|
In this post we discuss several recently published research reports suggesting that Parkinson’s disease may be an autoimmune condition. “Autoimmunity” occurs when the defence system of the body starts attacks the body itself. This new research does not explain what causes of Parkinson’s disease, but it could explain why certain brain cells are being lost in some people with Parkinson’s disease. And such information could point us towards novel therapeutic strategies. |

The first issue of Nature. Source: SimpleWikipedia
The journal Nature was first published on 4th November 1869, by Alexander MacMillan. It hoped to “provide cultivated readers with an accessible forum for reading about advances in scientific knowledge.” It has subsequently become one of the most prestigious scientific journals in the world, with an online readership of approximately 3 million unique readers per month (almost as much as we have here at the SoPD).
Each Wednesday afternoon, researchers around the world await the weekly outpouring of new research from Nature. And this week a research report was published in Nature that could be big for the world of Parkinson’s disease. Really big!
On the 21st June, this report was published:

Title: T cells from patients with Parkinson’s disease recognize α-synuclein peptides
Authors: Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Lindestam Arlehamn CS, Sette A
Journal: Nature. 2017 Jun 21. doi: 10.1038/nature22815.
PMID: 28636593
In their study, the investigators collected blood samples from 67 people with Parkinson’s disease and from 36 healthy patients (which were used as control samples). They then exposed the blood samples to fragments of proteins found in brain cells, including fragments of alpha synuclein – this is the protein that is so closely associated with Parkinson’s disease (it makes regular appearances on this blog).
What happened next was rather startling: the blood from the Parkinson’s patients had a strong reaction to two specific fragments of alpha synuclein, while the blood from the control subjects hardly reacted at all to these fragments.
In the image below, you will see the fragments listed along the bottom of the graph (protein fragments are labelled with combinations of alphabetical letters). The grey band on the plot indicates the two fragments that elicited a strong reaction from the blood cells – note the number of black dots (indicating PD samples) within the band, compared to the number of white dots (control samples). The numbers on the left side of the graph indicate the number of reacting cells per 100,000 blood cells.
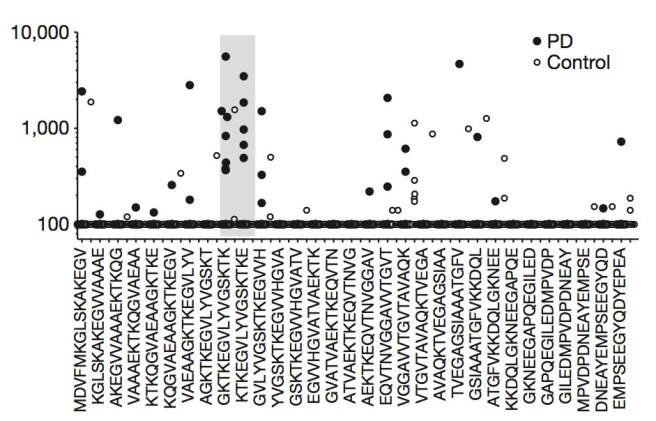
Source: Nature
The investigators concluded from this experiment that these alpha synuclein fragments may be acting as antigenic epitopes, which would drive immune responses in people with Parkinson’s disease and they decided to investigate this further.
What does antigenic epitopes mean?
It is probably best if we start with a bit of basic immunology.
Approximately 1/5 of all the cells in your body are involved in the immune system, which is responsible for defending you against substances that can make you sick. And usually those cells are really good – read: utterly ruthless and relentless – at protecting us against molecules that can cause infection and disease. They are particularly good at determining what is ‘self’ and what is not ‘self’ – that is to say, they can tell which substances are part of you (as an organism) and which are not. Not ‘self’ could simply be considered as anything that does not have an origin inside your body.
If the immune system is working correctly, when a pathogen (an agent that causes disease or damage) is detected in your body, it will quickly be determined to be not ‘self’. This judgement will be made by the identification of antigens on the surface of the pathogen. An antigen is defined as any substance or molecule that is capable of causing an immune response in an organism.
A good example of a pathogen is the common cold virus. Once inside the body, the presence of the virus will be detected by cells in the immune system and given that the virus will be presenting antigens on its surface that are clearly not self, an immune response will be initiated. The cells that carry out the immune response are white blood cells known as lymphocytes.

That big cell in the middle is a lymphocyte. Source: ASH
There are basically two types of immune response:
- An antibody response
- A cell-mediated immune response
These processes are carried out by two different types of lymphocytes (B cells and T cells). In the antibody response, B cells are activated and they begin to secrete Y-shaped proteins called antibodies. These are used by the immune system to label and neutralise foreign or dangerous substances.

Antibodies binding to a virus. Source: Biology-questions-and-answers
Antibodies bind to parts of the antigen called epitopes. Also known as antigenic determinants, an epitope is the part of an antigen that is recognised by an antibody. Antibodies by themselves can do a pretty good job of stopping pathogens, by blocking them from attaching to cells or by sticking together and clustering the antigens to prevent them from doing anything bad.

Antibody binding to antigens. Source: Venngage
Cell-mediated immunity, on the other hand, is an immune response that does not involve antibodies. This approach relies on antigen-presenting cells, cytotoxic T-cells, and the release of various cytokines in response to an antigen.
Que?
In cell-mediated immune response, when a foreign object (like a bacteria) enters the body it will be detected by what we call ‘antigen-presenting cells’ (such as macrophage cells). Upon detection, these brave, selfless little cells will engulf the bacteria and digest them into hundreds or thousands of antigen fragments. The macrophage cell will bundle these fragments together in what we call a major histocompatibility complex (MHC). The macrophage cell next displays this MHC on its own cell surface for other cells discover.

Source: Boundless
The ‘other’ cells are another type of lymphocyte called a T cell. T cells derive their name from the fact that they mature in the thymus. Once mature, they are released to do very specific tasks. Until they encounter an ‘antigen-presenting cells’, however, T-cells are as useless and witless as teenagers. They need to be stimulated by the ‘antigen-presenting cells’ before they know what they are going to do with their lives.
Once stimulated, there are basically two main types of T cells:
- helper T cells
- cytotoxic T cells
Helper T cells are nice and useful because they like to tell other immune cells about particular pathogens. Cytotoxic T cells, on the other hand, simply get on with the dirty job of killing off antigen presenting cells/bacteria/viruses/etc. And these brutal, heartless thugs do not discriminate – all they care about is whether a particular antigen is present or not.
As we suggested above, in order to do its job a mature T cell must encounter an antigen-presenting cell which will offer the T cell a particular MHC complex to inspect. This interaction will activate the T cell, and it also provides the T-cell a specific set of antigens to go looking for.
Once activated by an antigen-presenting cell, a cytotoxic T cells will begin creating many versions of itself (or clones) through a process of cell division (called mitosis). These clones will have one specific set of cell-surface receptors which will bind to the antigens in the MHC complex that was offered by the antigen-presenting cell. These “brutal, heartless thugs” will next go searching the body for anything that has the antigens it can bind to.

Source: Immuneresponse
Once these cloned cytotoxic T cells have identified something (cell, bacteria, virus, etc) that exhibits the antigens they are looking for they will begin the process of killing that thing. They do their killing by releasing signalling molecules (called cytokines) which encourage an antigen presenting bacteria or cell to undergo apoptosis (or programmed cell death). Some of the cytokines will also recruit other members of the immune system to come and help with the killing, and subsequent cleaning up of the mess.
Ok, so if a cell is presenting an antigen on its surface that a cytotoxic T cell is looking for then that cell could be in big trouble?
Exactly. Anything that is acting as an antigenic epitope for the cytotoxic T cell to bind to will increase the risk of driving an immune response.
So do the brain cells that are lost in Parkinson’s disease presenting these MHC complexes on their cell surface?
Excellent question.
There are actually different types of MHC complexes. The most common are MHC class I and MHC class II. MHC class I complexes are found on all cells except red blood cells, while MHC class II complexes are only found on the antigen-presenting cells. In the brain, MHC class I complexes are present during development, but their levels drop off as we age.
A few years ago, however, the researchers who conducted the study we are reviewing today, published data suggesting that the cells most affected in Parkinson’s disease may have higher levels of MHC class I complexes, which may be making them vulnerable:

Title: MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration.
Authors: Cebrián C, Zucca FA, Mauri P, Steinbeck JA, Studer L, Scherzer CR, Kanter E, Budhu S, Mandelbaum J, Vonsattel JP, Zecca L, Loike JD, Sulzer D
Journal: Nat Commun. 2014 Apr 16;5:3633.
PMID: 24736453 (This article is OPEN ACCESS if you would like to read it)
The investigators analysed human postmortem brain samples from people with and without Parkinson’s disease and they found that MHC class I complexes were present on many of the populations of neurons in the brain that are vulnerable to Parkinson’s disease (particularly the dopamine neurons in the substantia nigra and the norepinephrine producing neurons in an area called the locus coeruleus.
The investigators next conducted experiments in cell cultures using dopamine neurons that were made from human embryonic stem cells and they found that these cells were more susceptible to presenting MHC class I complexes when encouraged to than other types of neurons. The encouragement was caused by the activation of the helper cells in the brain called microglia. And the microglia were activated by exposure to alpha synuclein protein.
Thus, the researchers became interested in the idea that alpha synuclein from one dying cell could be activating microglia cells, which in turn makes other dopamine neurons present MHC class I complexes, making them vulnerable to inducing an immune response (Click here to read an OPEN ACCESS review of this topic by the investigators themselves).
And this was just a cute idea until the researchers published their results this week.
Which brings us back to the report again – there is a really interesting twist in it:

Title: T cells from patients with Parkinson’s disease recognize α-synuclein peptides
Authors: Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Lindestam Arlehamn CS, Sette A.
Journal: Nature. 2017 Jun 21. doi: 10.1038/nature22815.
PMID: 28636593
So the researchers observed T cells in the blood from people with Parkinson’s disease having a strong reaction to two specific fragments from alpha synuclein. These two fragments are from a region of the alpha synuclein protein called Y39. Interestingly, this Y39 region is very close to many of the genetic mutations in alpha synuclein that are associated with Parkinson’s disease (specifically A30P, E46K, A53T – in red on the left in the figure below).

Structure of alpha synuclein, showing mutation sites. Source: Frontiers
The researchers next looked at which MHC-associated proteins were responsible for putting the Y39 fragments into the MHC complexes for cell membrane presentation. The organising and presenting of these MHC complexes on the surface of a cell requires a lot of proteins all working together in perfect synchrony. The researchers found that the fragments were specifically displayed by two MHC class II proteins called HLA/DRB5*01:01 and HLA/DRB1*15:01,…
(and here comes the BIG twist)
…which if mutated are both associated with increased risk of developing Parkinson’s disease.

Title: Association of Parkinson disease with structural and regulatory variants in the HLA region.
Authors: Wissemann WT, Hill-Burns EM, Zabetian CP, Factor SA, Patsopoulos N, Hoglund B, Holcomb C, Donahue RJ, Thomson G, Erlich H, Payami H.
Journal: Am J Hum Genet. 2013 Nov 7;93(5):984-93.
PMID: 24183452 (This report is OPEN ACCESS if you would like to read it)
In this study, the investigators analysed the DNA from 2000 people with Parkinson’s disease and 1986 control subjects and they found that the risk of developing Parkinson’s disease was positively associated with variations in both HLA/DRB5*01:01 and HLA/DRB1*15:01 (in addition to other regions). Whereas approximately 15% of healthy control subjects carry variations in one of these genes, 1/3 of people with Parkinson’s disease have one of them.
So collectively these findings suggest that certain genetic variants in MHC-associated genes may cause particular fragments of alpha synuclein to be exposed in MHC complexes, causing T cells to mistakenly identify the alpha synuclein as a pathogen and thus trigger an autoimmune response that destroys any cell presenting alpha synuclein in MHC complexes. Likewise, the mutations in alpha synuclein which are located near the Y39 region and associated with Parkinson’s disease, could be causing this fragment to accidentally be exposed in MHC complexes (this needs to be further investigated though).
Source: Seekingalpha
The investigators are now seeking to discover whether the immune response provoked by alpha synuclein is a primary cause of Parkinson’s disease or whether it merely contributes to the brain cell death associated with the condition after the disease is triggered by something else. It is already apparent from the results of the study, however, that this ‘antigen presenting process’ is not going to explain every case of Parkinson’s disease, and the investigators acknowledge this. Hence the reason why the media headlines are reporting that autoimmunity may partly explain Parkinson’s disease.
In fact, only 40% of the blood from people with Parkinson’s disease in the study exhibited immune responses to the alpha synuclein fragments, and this may reflect differences between the participants in the study, particularly with regards to genetic variations. For example, last year a research report was published in the journal Cell that identified Parkinson’s associated proteins PINK1 and Parkin as suppressors of an immune response eliciting pathway (Click here to read more about that study).
That study found that in the absence of PINK1 or Parkin fragments of mitochondria (the power stations of cells) could be presented in MCH class I complexes, which would result in an immune response. PINK1 and Parkin are both involved in the normal removal of unhealthy mitochondria (Click here and here to read more about this). Without PINK1 or Parkin, old and dysfunctioning mitochondria start piling, making the cell sick. Thus, it may that people with PINK1 or Parkin genetic mutations may have an autoimmune component to their disease (perhaps falling into ‘the 40%’), while other people with Parkinson’s disease who don’t have these sorts of genetic variants will have alternative explanations to explain their condition.
The research groups that conducted the study we are reviewing today are now recruiting and analysing additional participants (with and without Parkinson’s disease), and are working to identify the molecular steps that lead to the autoimmune response in animal and cellular models.
What exactly is an autoimmune response?
An autoimmune response is an immune response within an organism against its own healthy cells and tissues. Any disease that results from such an immune response is called an autoimmune disease.

Different types of autoimmune diseases. Source: DrJockers
So is Parkinson’s disease an autoimmune disease?
For a condition to be considered an autoimmune disease it needs to conform to what is called Witebsky’s postulates (first formulated by Ernest Witebsky and associates in 1957; though they were modified in 1994). An autoimmune disease must show:
- Direct evidence from transfer of disease-causing antibody or disease-causing T lymphocyte white blood cells
- Indirect evidence based on reproduction of the autoimmune disease in experimental animals
- Circumstantial evidence from clinical clues
- Genetic evidence suggesting “clustering” with other autoimmune diseases
The research report we have reviewed in this post provides us with evidence of the first requirement. We also have evidence of the second requirement – Click here to read more about this. For the final two requirements, there has been an ever increasing number of reports regarding associations between Parkinson’s disease and other autoimmune diseases.
In fact, just this month alone we have had two separate studies published: one suggesting that naturally occurring “autoantibodies” (which play an important role in clearing and blocking circulating ‘self’ proteins) are lower in people with Parkinson’s disease than healthy control subjects. The second study presents strong evidence that Parkinson’s disease shares a number of genetic associations with autoimmune diseases.
Here is the first study:

Title: Autoimmune antibody decline in Parkinson’s disease and Multiple System Atrophy; a step towards immunotherapeutic strategies.
Authors: Brudek T, Winge K, Folke J, Christensen S, Fog K, Pakkenberg B, Pedersen LØ.
Journal: Mol Neurodegener. 2017 Jun 7;12(1):44.
PMID: 28592329 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers collected blood samples from samples from 46 people with Parkinson’s disease, 18 people with Multiple System Atrophy (a condition very similar to Parkinson’s disease), and 41 healthy control subjects. When they analysed the blood for autoantibodies targeting the alpha synuclein protein, they found reduced levels in people with Parkinson’s disease when compared to healthy controls, and even more reduced in people with Multiple System Atrophy. The researchers concluded that reduced levels of these antibodies for alpha synuclein results in more alpha synuclein floating around and causing an inflammatory environment. They also propose that the results provide a good rationale for testing immune-based therapeutic strategies directed against pathological alpha synuclein (such as the Affiris and Prothena clinical trials we have previously discussed – click here to read more about this).
The second report is a much larger study:

Title: Genome-wide Pleiotropy Between Parkinson Disease and Autoimmune Diseases
Authors: Witoelar A, Jansen IE, Wang Y, Desikan RS, Gibbs JR, Blauwendraat C, Thompson WK, Hernandez DG, Djurovic S, Schork AJ, Bettella F, Ellinghaus D, Franke A, Lie BA, McEvoy LK, Karlsen TH, Lesage S, Morris HR, Brice A, Wood NW, Heutink P, Hardy J, Singleton AB, Dale AM, Gasser T, Andreassen OA, Sharma M; International Parkinson’s Disease Genomics Consortium (IPDGC), North American Brain Expression Consortium (NABEC), and United Kingdom Brain Expression Consortium (UKBEC) Investigators.
Journal: JAMA Neurol. 2017 Jun 5. doi: 10.1001/jamaneurol.2017.0469.
PMID: 28586827
In this study, the researchers analysed DNA collected from 138 511 individuals of European ancestry and they identified 17 novel genetic loci shared between Parkinson disease and a series of autoimmune conditions (including type 1 diabetes, Crohn disease, ulcerative colitis, rheumatoid arthritis, celiac disease, psoriasis, and multiple sclerosis). According to this study, apparently healthy individuals with a lot of these shared genetic variants which predisposes them to inflammation conditions, could also be at increased risk for developing Parkinson’s disease.
Man, this all sounds really bad. What can we do about it?
Well firstly, we can start by not considering these results as bad news.
In fact, these studies could represent a major step forward in the right direction for a lot of people with Parkinson’s disease. These research findings are extremely useful for us.
As one of the investigators in the blood study, Dr. Alessandro Sette, (from the Centre for Infectious Disease in La Jolla, Calif.) has suggested the findings “raise the possibility that an immunotherapy approach could be used to increase the immune system’s tolerance for alpha synuclein, which could help to ameliorate or prevent worsening symptoms in Parkinson’s disease patients,”.
Dr. Sette also adds that “These findings could provide a much-needed diagnostic test for Parkinson’s disease, and could help us to identify individuals at risk or in the early stages of the disease.”
And the authors of the study also point towards drugs that could be applied to individuals that fit a potential autoimmune criteria for Parkinson’s disease. For example, they mentioned in the research article that candesartan cilexetil – a drug used for hypertension – was recently shown to reduce the activation microglia (the helper cells in the brain) which is caused by a build up of the Parkinson’s associated protein alpha synuclein:

Title: Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders.
Authors: Daniele SG, Béraud D, Davenport C, Cheng K, Yin H, Maguire-Zeiss KA.
Journal: Sci Signal. 2015 May 12;8(376):ra45.
PMID: 25969543 (This article is OPEN ACCESS if you would like to read it)
This study found that candesartan cilexetil reversed the activation of microglia exposed to alpha synuclein, supporting the possibility of repurposing this drug for conditions like Parkinson’s disease. And other research groups have also reported neuroprotective effects of candesartan cilexetil in models of Parkinson’s disease (Click here to read more on this). Candesartan (also known by trade names such as Blopress, Atacand, Amias, and Ratacand) is an angiotensin II receptor antagonist used mainly for the treatment of hypertension.

Candesartan. Source: Wikipedia
In addition to pointing us towards novel therapy options, the new results suggesting an autoimmune component to some people with Parkinson’s disease would also represent a tremendous boost of support for therapies that are currently being clinically tested. Specifically those trials that are focused on suppressing the immune system, such as the immunomodulation study being conducted in Nebraska – a clinical trial of the drug Sargramostim in Parkinson’s disease.

Nebraska. Source: The Toast
Sargramostim stimulates regulatory T (Treg) cells. Treg cells are an important part of the immune system that we haven’t discussed in this particular post. They basically maintain law and order in the immune system. They do this by enforcing a dominant negative regulation on other immune cells, particularly other T-cells. Think of T-cells as the inquisitive neighbours curious about and snooping around a local crime scene, and then imagine that Treg cells are the police telling them “nothing to see here, move along”.

Tregs maintaining order. Source: Keywordsuggestions
Treg cells are particularly important for calming down Helper T cells and Cytotoxic T cells (often referred to in combination as T-effectors). The normal situation in your body is to have a balance between Helper T cells/Cytotoxic T cells and Treg cells. If there are too many excited Helper T cells and Cytotoxic T cells, there is increased chances of things going wrong and autoimmunity occurring.

A delicate balance between healthy and autoimmune disease. Source: Researchgate
Thus, treatments that suppress the T-effectors may be very useful in slowing down a condition like Parkinson’s disease (particularly if autoimmunity is a component of this condition). One caveat here, however, is that too many Treg cells is not a good situation either, as they can leave the immune system too suppressed and individuals vulnerable to other diseases. A delicate balancing act will be required for such a treatment approach. To read more about the Sargramostim clinical trial in Nebraska, please click here to see our post about it.
So what does it all mean?
Phew! Long post.
It is basically the combination of three posts, each dealing with separate autoimmunity research reports associated with Parkinson’s disease,… plus a lesson in elements of basic immunology (which dragged on a bit). But I felt the research results are really important and the topic deserved to be done as one big post. Understand this: collectively, the research may represent a major turning point for the Parkinson’s disease community.
The research suggests that Parkinson’s disease may – at least partly – be an autoimmune condition. If further investigations (including replication of these original results by independent research groups) supports the idea that some people with Parkinson’s disease have an autoimmune component to their condition, this knowledge will provide us with a starting point to begin dividing the affected individuals into groups which could be better treated by the use of therapies oriented towards autoimmunity.
Ultimately any cure of this condition will probably utilise multiple treatments (eg. something to slow the progress of the disease, something protect cells from dying, something to distract the immune system, and something to begin replacing the cells that have been lost). Key to that future is a better understanding of the various components underlying the disease. The potential discovery of an autoimmune component to Parkinson’s disease in some people may help in the personalisation of therapy.
We are really excited by this.
EDITORIAL NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. While many of the drugs/treatments discussed on the website are clinically available, they can have significant side effects and may affect the efficacy of other treatments. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Niaid
I am realizing how much I don’t know about all this. Thanks for informing us.
LikeLike
Excellent article Simon – yes I know it is a tad long-winded but there is no other way of getting the message across that PD is very complex and (I agree 100% with you) the achievement of near-total control of PD will require more than one medication. I think 3 or 4 drugs will figure and lifestyle change to make nutrition and exercise appropriate to the type of PD will also count for a bit. Also, exercise as in the form of brain training will assume greater importance IF things like Parky psychosis and depression continue to persist despite the new medications stopping decay and maybe even rebuilding lost parts of the brain.
LikeLike
Excellent Thank you very mcuh
LikeLike
Hi Harry,
Thanks for the comment. Glad you found the post interesting.
Kind regards,
Simon
LikeLike
Simon,
Great blog and post and service to the community.
My question is – does the research suggest two different, opposing views, that:
The immune system defenses against α-synuclein should be enhanced (e.g., Affiris, Prothena PRX002, Biogen BIIB054) or the immune system response should by suppressed (e.g., Sargramostim)?
Or is it a case of: ‘it depends” or ‘a combination of both’?
Am I missing something?
LikeLike
Hi KJJ,
Thanks for the interesting comment – glad you liked the post. If the results of this study can be independently replicated, I suspect the answer will fall into both the “it depends” and the “a combination of both” baskets and I’m not trying to avoid the question with this vague answer. Let me explain:
“It depends” because 1.) an important detail in this study is that only 40% of the blood samples reacted to these two regions of alpha synuclein. Thus, there may be a large group within the PD affected community for whom this whole antigenic concept may not relevant. That is to say, some other agent (toxin, pathogen, etc) may be causing the brain cells to die in those folks rather than an autoimmune situation (Alternatively, perhaps the immune system of the other 60% reacts to different Parkinson’s associated proteins acting as antigenic epitopes – something other than these two pieces of alpha synuclein). This all still needs to be determined.
Also “It depends” because 2.) the key question in the Affiris, Prothena, and Biogen trials now is which epitope/s are the antibodies actually binding to? They all have a preference for the aggregated form of alpha synuclein, but within that structure which epitopes are they specifically targeting? Clearing alpha synuclein floating between cells may slow down/halt the spread of the disease, but if these antibodies are also binding to a piece of MHC complex being presented by some of the vulnerable neurons within the brain, then this might lead to a bad situation. One precaution for this scenario would be to simply take blood samples from folks being recruited to these trials and screening them across various alpha synuclein peptides. If a blood sample reacts, perhaps that individual should be excluded from the study for safety reasons. The basic hypothesis of the clinical trial can still be tested, without the confounding element of an untended negative effect. But again, independent replication of the antigenic epitope results is required before we go wandering too far down this path.
And it’s “a combination of both” because I suspect that the immunomodulation therapy (eg. Sargramostim, etc) will only work if done in combination with an immunotherapy (eg. antibody/vaccine) type of approach. If you do not slow/halt the spread of the disease, immunomodulation will simply hold the “thugs of the immune system” at bay, while the disease continues to spread unabated. Using both of these types of therapies, however, will be a delicate balancing act.
Thanks again for asking the question – hope all of this makes sense. I was really trying to avoid the vaccine/immunotherapy topic in the post 🙂 but I guess by not dealing with it, I made it an obvious question to ask!
Kind regards,
Simon
LikeLike
Recent interesting Parkinson’s/autoimmune finding (possibly suggesting psoriasis meds for Parkinson’s?):
FAU researchers find possible cause of Parkinson’s disease in the patients’ immune system…antibodies which block the effect of Th17 cells, including one antibody which is already being used on a daily basis in the hospital to treat psoriasis, were able to largely prevent the death of the nerve cells. see:
https://www.news-medical.net/news/20180719/FAU-researchers-find-possible-cause-of-Parkinsone28099s-disease-in-the-patientse28099-immune-system.aspx
Note that this article -> https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4817438/
appears to suggest a link between psoriasis and Parkinson’s:
LikeLike
Hi KJJ_me,
Thanks for the links. It is really interesting research. So much so that:
Hope it all makes sense – it was a little rushed.
Kind regards,
Simon
LikeLike
Hi Simon,
Great blog, Real practical value.
Now 2 blood pressure meds help with different aspects PD:
Isradipine, calcium channel blocker
Candesartan, a triple threat: blood pressure, PD and extends life span in rats due to blocking Angiotensin 2.
LikeLike