|
This week a group of scientists have published an article which indicates differences between mice and human beings, calling into question the use of these mice in Parkinson’s disease research. The results could explain way mice do not get Parkinson’s disease, and they may also partly explain why humans do. In today’s post we will outline the new research, discuss the results, and look at whether Levodopa treatment may (or may not) be a problem. |
The humble lab mouse. Source: PBS
Much of our understanding of modern biology is derived from the “lower organisms”.
From yeast to snails (there is a post coming shortly on a snail model of Parkinson’s disease – I kid you not) and from flies to mice, a great deal of what we know about basic biology comes from experimentation on these creatures. So much in fact that many of our current ideas about neurodegenerative diseases result from modelling those conditions in these creatures.
Now say what you like about the ethics and morality of this approach, these organisms have been useful until now. And I say ‘until now’ because an interesting research report was released this week which may call into question much of the knowledge we have from the modelling of Parkinson’s disease is these creatures.
You see, here’s the thing: Flies don’t naturally develop Parkinson’s disease.
Nor do mice. Or snails.
Or yeast for that matter.
So we are forcing a very un-natural state upon the biology of these creatures and then studying the response/effect. Which could be giving us strange results that don’t necessarily apply to human beings. And this may explain our long history of failed clinical trials.
We work with the best tools we have, but it those tools are flawed…
What did the new research report find?
This is the study:

Title: Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease
Authors: Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, Santos DP, Blanz J, Obermaier CD, Strojny C, Savas JN, Kiskinis E, Zhuang X, Krüger R, Surmeier DJ, Krainc D
Journal: Science, 07 Sept 2017 – Early online publication
PMID: 28882997
The researchers who conducted this study began by growing dopamine neurons – a type of cell badly affected by Parkinson’s disease – from induced pluripotent stem (IPS) cells.
What are induced pluripotent stem cells?
This is Prof Shinya Yamanaka:

Prof Shinya Yamanaka. Source: Glastone Institute
He’s a rockstar in the scientific research community.
Prof Yamanaka is the director of Center for induced Pluripotent Stem Cell Research and Application (CiRA); and a professor at the Institute for Frontier Medical Sciences at Kyoto University.
In 2006, his research group published a report demonstrating how someone can take a skin cell and re-program it so that it becomes a stem cell – a cell capable of becoming any kind of cell in the body.
Here’s that study:

Title: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors.
Authors: Takahashi K, Yamanaka S.
Journal: Cell. 2006 Aug 25;126(4):663-76.
PMID: 16904174 (This article is OPEN ACCESS if you would like to read it)
Shinya Yamanaka‘s team started with the hypothesis that a gene – the section of DNA that provides the instructions for making a particular protein – which is important to the maintenance of embryonic stem cells (the cells that give rise to all cells in the body) might also be able to cause an embryonic-like state in mature adult cells. They selected twenty-four genes that had been previously identified as important in embryonic stem cells to test this idea. They used re-engineered retroviruses to deliver these genes to mouse skin cells. The retroviruses were emptied of all their disease causing properties, and could thus function as very efficient biological delivery systems.
The skin cells were engineered so that only cells in which reactivation of the embryonic stem cells-associated gene, Fbx15, would survive the testing process. If Fbx15 was not turned on in the cells, they would die. When the researchers infected the cells with all twenty-four embryonic stem cells genes, remarkably some of the cells survived and began to divide like stem cells.
In order to identify the genes necessary for the reprogramming, the researchers began removing one gene at a time from the pool of twenty-four. Through this process, they were able to narrow down the most effective genes to just four: Oct4, Sox2, cMyc, and Klf4, which became known as the Yamanaka factors.
This new type of cell is called an induced pluripotent stem (IPS) cell – ‘pluripotent’ meaning capable of any fate.
Making IPS cells. Source: learn.genetics
Induced pluripotent stem cell are stem cells – ‘pluripotent’ meaning capable of any fate – that have been derived from an adult, mature cell (usually skin cells). The adult cell has been ‘induced’ into being a stem cell, by the reprogramming process. The discovery of these cells means that patient-specific tissue can be generated in petri dishes (or cell culture) and investigated experimentally – the underpinnings of personalised medicine.
Ok, so the researchers used these IPS cells to make dopamine neurons?
Exactly.
They took skins cells from people with different types of Parkinson’s disease (based on different genetic mutations associated with the condition) and with a little bit of biological magic they made IPS cells, which were then grown into dopamine neurons. These cells from different types of Parkinson’s disease included skin cells from people with genetic mutations in the DJ-1 gene (Click here to learn more about DJ-1), the Parkin gene, the PINK1 gene (Click here and here to learn more about Parkin and PINK1), and the LRRK2 gene (Click here to learn more about LRRK2).
The researchers started with the cells that have DJ-1 mutations. After 50 days of growing these cells, the investigators noticed that the cells had very high levels of oxidative stress. DJ-1 is present in almost all cells, including those of the brain. And it is a busy protein, involved in many different roles required for normal biological activity. One of the primary functions of DJ-1, however, is to help protect cells from oxidative stress, so it is no real surprise that there is an increase in levels of oxidative stress.
What is oxidative stress?
Oxidative stress is the damage done to a cell by the process of oxidation.
And yes, I know what you are going to ask next: What is oxidation?
Oxidation is the loss of electrons from a molecule, which in turn destabilises that particular molecule. Think of iron rusting. Rust is the oxidation of iron – in the presence of oxygen and water, iron molecules will lose electrons over time. Given enough time, this results in the complete break down of objects made of iron.

Rusting iron. Source: Thoughtco
The exact same thing happens in biology. Molecules in your body go through a similar process of oxidation – losing electrons and becoming unstable. This chemical reaction leads to the production of what we call free radicals, which can then go on to damage cells.
What is a free radical?
A free radical is an unstable molecule – unstable because they are missing electrons. They react quickly with other molecules, trying to capture the needed electron to re-gain stability. Free radicals will literally attack the nearest stable molecule, stealing an electron. This leads to the “attacked” molecule becoming a free radical itself, and thus a chain reaction is started. Inside a living cell this can cause terrible damage, ultimately killing the cell.
Antioxidants are the good guys in this situation. They are molecules that neutralize free radicals by donating one of their own electrons. The antioxidant don’t become free radicals by donating an electron because by their very nature they are stable with or without that extra electron.

How free radicals and antioxidants work. Source: h2miraclewater
Ok, got it. The DJ-1 cells had high levels of oxidative stress. Did the researchers notice anything else?
Yes, the DJ-1 cells also exhibited the accumulation dense clustering of neuromelanin. And before you asks: Neuromelanin is the brain version of a protein called melanin, which is a pigment found in the skin, eyes, and hair. In the brain, certain types of cells, such as the dopamine neurons, produce neuromelanin.

Neuromelanin (brown) in dopamine neurons. Source: Schatz
Neuromelanin is produced from the oxidation of dopamine. Thus, a gradual build up of neuromelanin in the DJ-1 dopamine neurons indicates an increase in the levels of oxidised dopamine.
And this is exactly what the researchers found: increased levels of oxidised dopamine. And the levels of oxidised dopamine progressively increased in the DJ-1 dopamine neurons from day 70 in cell culture to day 150. But here’s the interesting thing: The same increase in oxidised dopamine was not observed in dopamine neurons made from IPS cells from healthy control subjects. This effect was specific to the DJ-1 cells.
That was until the researchers looked at dopamine neurons made from IPS cells collected from people with ‘idiopathic’ Parkinson’s disease (idiopathic meaning that no genetic mutation is involved). These dopamine neurons ALSO exhibited increased levels in oxidised dopamine levels, but only at later time points (between day 150 and day 180 in cell culture).
Neurons from IPS cells. Source: Reprocell
Now, neuromelanin is often found in structures called lysosomes. Lysosomes are small bags of digestive enzymes that can be found inside cells. They help to break down proteins like neuromelanin that have served their function and need to be digested and disposed of (or recycled).

How lysosomes work. Source: Prezi
Inside the lysosomes are enzymes (such as glucocerebrosidase) which help to break material down into useful or disposable parts. The lysosome will fuse with other small bags (called vacuole) which act as storage vessels of food or waste inside a cell. The enzymes from the lysosome will mix with the material in the vacuole and digest it (or it break down into more manageable components).
Given the increase in neuromelanin levels in the DJ-1 dopamine neurons, the researchers wondered if the lysosomal recycling function was impaired in these cells, but further analysis suggested no detectable impairment. What they did find, however, was a decrease in the activity of the lysosomal enzyme glucocerebrosidase at day 70. This decrease was specific to glucocerebrosidase – there was no drop in the activity of other lysosomal enzymes – and the dopamine neurons from IPS cells from idiopathic Parkinson’s disease also exhibited a delayed decrease in glucocerebrosidase activity (starting at day 180 in cell culture).
Why is gluco-cere-bro-si-dase important?
Genetic mutations in the gene that provides the instructions for the production of this enzyme (the gene is called GBA) are the most common genetic variation that are associated with increased risk of developing Parkinson’s disease.
And this is where the story starts to get interesting:
When the researchers next looked at dopamine neurons made from IPS cells from people with other Parkinson’s associated genetic mutation (such as Parkin, PINK1, and LRRK2 – all mentioned above), they found a similar increase in oxidised dopamine across all of the cells, as well as decreased glucocerebrosidase activity – particularly in Parkin mutant dopamine neurons.
These results suggested that to the researchers that oxidative stress directly modifies and disrupts glucocerebrosidase enzymatic activity.
Wow. So could the researcher treat the cells with anything to correct this?
Yes indeed, and that is exactly what they did next. They tested several different treatments.
First, they treated the cells with Acetylcysteine (also known as N-acetylcysteine or simply NAC). I have discussed NAC in a previous post (Click here to read that post).
 Acetylcysteine. Source: Wikimedia
Acetylcysteine. Source: Wikimedia
NAC (commercially named Mucomyst) is a prodrug – that is a compound that undergoes a transformation when ingested by the body and then begins exhibiting pharmacological effects. Acetylcysteine serves as a prodrug to a protein called L-cysteine, and – just as L-dopa is an intermediate in the production of dopamine – L-cysteine is an intermediate in the production of another protein called glutathione.
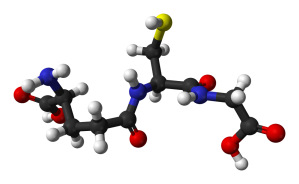
Glutathione. Source: Wikipedia
Glutathione (pronounced “gloota-thigh-own”) is a potent antioxidant. It is produced naturally in nearly all cells. In the brain, glutathione is concentrated in the helper cells (called astrocytes) and also in the branches of neurons, but not in the actual cell body of the neuron.
When the researcher treated the DJ-1 mutant cells with NAC, it stopped the accumulation of oxidised dopamine and improved lysosomal glucocerebrosidase activity. The Parkinson’s disease-associated protein alpha synuclein was also found to have elevated levels in the DJ-1 mutant cells and NAC treatment also found to have a beneficial effect on reducing levels of this protein.
Next, the researchers treated the cells with a calcium channel antagonist called ‘Isradipine’. I have been meaning to write a post about this drug for a while because there is a big clinical trial ongoing for Isradipine in Parkinson’s disease – it is called STEADY-PD III (Click here to learn more about it).
Source: STEADY-PD III
Isradipine (tradenames DynaCirc, Prescal) is a calcium channel blocker, which is a class of drugs usually prescribed for the treatment of high blood pressure (and thus reducing the risk of stroke or heart attack). Calcium channels are found in the membrane of excitable cells (such as muscle, glial cells, neurons, etc.).
Isradipine. Source: Dailymed
The concentration of calcium outside the cell is normally several thousand times higher than inside the cell. Activation of calcium channels allows calcium to rush into the cell and makes the cell excited. Prof Frank Church over at the ‘Journey with Parkinson’s’ blog has a great page on Isradipine (Click here to read that post).
Calcium getting cells excited. Source: STEADY-PD III
Increased calcium channel activation is also known to enhance oxidant stress and dopamine synthesis. When the researchers tested Isradipine on the dopamine neurons in cell culture, they found that it significantly decreased the accumulation of oxidised dopamine.
Thus, the researchers were able to interupt the toxic cascade that was affecting these dopamine neurons… in cell culture at least.
Does the effect work in mouse models of these genetic forms of Parkinson’s disease?
Ok, so this is where this research report is extremely interesting.

A lab mouse. Source: USNews
The investigators next examined whether the toxic cascade of events that they observed in the human dopamine neurons grown in cell culture also occurs in mouse models of Parkinson’s disease. They started by looking at the dopamine neurons in mice lacking the DJ-1, and they found….
Nothing.
What-so-ever.
There was no increase in the amounts of oxidised dopamine (at both 3 months or 12 months of age). There was also no increase in the amount of alpha synuclein in these mice. AND levels of glucocerebrosidase activity were normal. On top of all of this, they observed no dopamine neuron degeneration in these mice (which is in agreement with previous research – click here to read that report).
Strange, huh?
The researchers were left scratching their heads for a moment. But then they decided to model Parkinson’s disease in these mice in a more disease-relevant way. They figured that since mutations in the alpha synuclein gene results in the accumulation of alpha synuclein protein in a genetic form of Parkinson’s disease, they would increase levels of mutant alpha synuclein protein in the DJ-1 mutant mice and see what impact this has on oxidised dopamine levels.
And guess what happened?
Suddenly the DJ-1 mutant mice (that had an over production of alpha synuclein) had elevated levels of oxidised dopamine and decreased lysosomal glucocerebrosidase activity!
Is it just increased levels of alpha synuclein that can cause these problems?
No.
The researchers also investigated whether simply increasing dopamine levels might also trigger problems in the DJ-1 mutant mice. They started feeding the mice with Levodopa-supplemented food to the mice, and………..this led to oxidised dopamine accumulation in the dopamine neurons as well. Levodopa treatment of the DJ-1 mutant mice (but not normal mice) also disrupted glucocerebrosidase enzymatic activity, increased alpha synuclein levels, AND caused the loss of dopaminergic neurons. Plus, the investigators repeated this experiment in cell culture with dopamine neurons from DJ-1 mutant mice and they found the same results.
Oh boy, so Levodopa can cause an increase in oxidised dopamine?
According to the results of this study report, yes.
And human dopamine neurons demonstrated higher levels of dopamine when compared to their mouse equivalent, suggesting a difference in dopamine production between the two species. Supporting this idea, the researchers found that Levodopa treatment was sufficient to increase oxidised dopamine in dopamine neurons made from IPS cells from healthy control humans, but this was not possible in dopamine neurons made from IPS cells from normal mice.
Thus (again, according to the results of this study) Levodopa treatment in humans appears to increase levels of oxidised dopamine.
Oh’ll man, this is bad! L-dopa increases the level of oxidised dopamine! I should stop my L-dopa treatment to lower my levels of oxidised dopamine!
No, you shouldn’t.
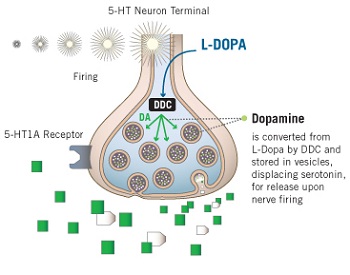
By the time someone is diagnosed with Parkinson’s disease, 50% of the dopamine neurons in the substantia nigra have been lost. When Levodopa treatment is initiated, the remaining dopamine neurons may take up some of the medication, but in a lot of cases other cells in the brain will also take up the Levodopa and produce dopamine. The enzyme that converts Levodopa into dopamine is called aromatic amino-acid decarboxylase (or AADC). This enzyme is not specific to dopamine neurons, it is found in many different cells including glia cells and serotonin neurons. Most of these cells are better able to handle oxidative stress than the dopamine neurons.
Serotonin (5HT) neuron producing dopamine from L-dopa. Source: galoncockwould
If you are concerned about these findings, however, please discuss them with your physician before making any changes to your treatment regime. Alternative treatments that do not cause excess levels of oxidative dopamine (such as dopamine agonists) are available. But I would like to add that this study needs to be independently replicated before we can consider the results validated. And clinical studies of dopamine agonists have demonstrated limited disease modifying potential of the drugs on Parkinson’s disease (Click here to read more about this), so it is unlikely that this oxidised dopamine idea is the cause of disease progression in Parkinson’s.
So what does it all mean?
From “To a Mouse,” by Robert Burns comes the line “The best laid schemes o’mice an’ men/Gang aft agley”, which was used in the title of John Steinbeck’s classic ‘Of mice and men’. It seemed appropriate here because this new research suggests that our efforts to use lower species to help explain our ailments ‘gang aft agley’.
Source: HMS
Having said that, if the researchers behind the report we reviewed today had not alternated between mice and human cells, they would not have observed the differences that they found.
The research report we have reviewed today raises concerns about comparing experimental results observed in mice with disease-associated phenomenon in humans. The investigators found that a toxic cascade of oxidised dopamine build up and reduction of enzymatic recycling of the dopamine causes problems in human cells, but not mouse cells – unless those cells are over supplied with Levodopa.
Whether oxidised dopamine is playing a role in the dysfunction and death of dopamine neurons in Parkinson’s disease, is yet to be determined. While the results of this new study are very interesting and point towards the beneficial use of antioxidants and calcium channel blockers, we need independent replication of the results to confirm the findings. It would also be interesting to conducted an analysis of clinical notes comparing outcomes between people treated with Levodopa and dopamine agonists to assess whether any ‘oxidised dopamine’ effect could be speculated upon in the clinic.
As always with interesting new research, the current study has raised many new questions. I look forward to seeing answers to those questions.
The banner for today’s post was sourced from Pinterest







Great post thought-provoking
LikeLike
Hi Mary,
Thanks for your comment – glad you found the post interesting.
Kind regards,
Simon
LikeLike
I have for a long time been suspicious about the applicability of rodent , and even primate, experiments to humans but aren’t these always checked out before any conclusions are drawn?
Keith
LikeLike
Hi Keith,
The rodent situation gets even more complicated as different strains can present very different ‘phenotypes’ – the physical features presented after a biological/genetic manipulation. It just goes to show that we have to be very careful shifting conclusions between species.
Kind regards,
Simon
LikeLike
My simpleton reaction – Seems like a breakthrough finding for research – which will take some time to reach patients down stream. First do no harm.
LikeLike
Indeed Dkdc!
LikeLike
I guess it does encourage the idea that olive leaf, resveratrol and other things Simon has written about that fight oxidation and inflammation are in fact helpful.
LikeLike
Hi Dkdc,
Antioxidants are a good idea for everyone as we age. But the current research report certainly supports the idea of a big double-blind, placebo-controlled study of NAC for PD, which the Cure PD Trust are currently setting up ( https://www.cureparkinsons.org.uk/the-nac-trial-the-facts). It’ll be interesting to see the results of this and the STEADY-PD trial.
Kind regards,
Simon
LikeLike
Seems like OTC NAC is not useful. We need it in syringes. But we have other things.
LikeLike
Not necessarily. I recently made an addendum to the NAC post published earlier this year (see the bottom of the post on this page: https://scienceofparkinsons.com/2017/07/06/glutathione-getting-the-knack-of-parkinsons-disease/). New research mentioned in that addendum suggests that not much oral NAC is actually required for beneficial levels to be achieved in the brain. This is the original research mentioned (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4720670/). Thus, I think NAC is worthy of another large clinical study – thus far the group sizes in the clinical studies have been very small. Dr Laurie Mischley at Bastyr University has previously conducted intranasal (delivery via nasal spray) clinical studies of NAC and she would be worth talking to if you are interested in NAC.
Kind regards,
Simon
LikeLike
Thanks
LikeLike
Great post!
I suppose with a perfect model we’d have all the answers already.
LikeLike