|
Gene therapy involves treating medical conditions at the level of DNA – that is, altering or enhancing the genetic code inside cells to provide therapeutic benefits rather than simply administering drugs. Usually this approach utilises specially engineered viruses to deliver the new DNA to particular cells in the body. For Parkinson’s, gene therapy techniques have all involved direct injections of these engineered viruses into the brain – a procedure that requires brain surgery. This year, however, we have seen the EXTREMELY rapid development of a non-invasive approach to gene therapy for neurological condition, which could ultimately see viruses being injected in the arm and then travelling up to the brain where they will infect just the desired population of cells. Last week, however, this approach hit a rather significant obstacle. In today’s post, we will have a look at this gene therapy technology and review the new research that may slow down efforts to use this approach to help to cure Parkinson’s. |
Gene therapy. Source: rdmag
When you get sick, the usual solution is to visit your doctor.
They will prescribe a medication for you to take, and then all things going well (fingers crossed/knock on wood) you will start to feel better. It is a rather simple and straight forward process, and it has largely worked well for most of us for quite some time.
As the overall population has started to live longer, however, we have begun to see more and more chronic conditions which require long-term treatment regimes. The “long-term” aspect of this means that some people are regularly taking medication as part of their daily lives. In many cases, these medications are taken multiple times per day.
A good example of this is Levodopa (also known as Sinemet or Madopar) which is the most common treatment for the chronic condition of Parkinson’s disease.
When you swallow your Levodopa pill, it is broken down in the gut, absorbed through the wall of the intestines, transported to the brain via our blood system, where it is converted into the chemical dopamine – the chemical that is lost in Parkinson’s disease. This conversion of Levodopa increases the levels of dopamine in your brain, which helps to alleviate the motor issues associated with Parkinson’s disease.

Levodopa. Source: Drugs
This pill form of treating a disease is only a temporary solution though. People with Parkinson’s – like other chronic conditions – need to take multiple tablets of Levodopa every day to keep their motor features under control. And long term this approach can result in other complications, such as Levodopa-induced dyskinesias in the case of Parkinson’s.
Yeah, but is there a better approach?
Some researchers (including yours truly) believe there is.
But we are not quite there yet with the application of this alternative approach. Let me explain:
For a long time now, researchers have been trying to apply something called “gene therapy” to Parkinson’s (and other medical conditions). Gene therapy basically involves introducing a new piece of DNA or replacing a faulty piece of DNA within a population of cells. DNA, as you may remember from high school science class provides the instructions for making proteins in the a cell and these proteins are the bits that actually do stuff.
By introducing a new piece of DNA into a cell, the cell can start to produce a functioning protein that it may not normally produce. In some diseases, a cell may produce a particular protein, but because the genetic instructions in the DNA (a section of the DNA called a gene) for that protein have a small error (a genetic mutation), a non-functioning version of the protein is actually being produced. The introduction of the new correct (functioning) version of that piece of DNA (or gene) into a cell can start the production of a functional version of the protein.
Gene therapy. Source: yourgenome
Alternatively, a gene can be introduced into a cell which would cause the cell to produce a protein that that cell usually does not produce. This sort of approach is being used in gene therapy for cancer, where ‘suicide genes’ are being introduced into cancer cells. These cause the cancer cell to die, by initiate an auto-destruct sequence resulting in cell death (a process called apoptosis). Another approach the cancer field is using is introducing a gene into cancer cells that cause a protein to be produced on the surface of the cancer cell that attracts the attention of the immune system. This ‘marker gene’ causes the immune system to attack the cancer cell, resulting in the death of the cancer cell.
Source: yourgenome
Cool. But what about Parkinson’s?
A lot of gene therapy has been tested in Parkinson’s research. And some of it has been tested in the clinic. A good example of the gene therapy approach for Parkinson’s is Oxford BioMedica‘s product called ‘Prosavin’ (or OXB-101).

Source: Oxford BioMedica
Prosavin is a virus that was injected directly into the brains of people with Parkinson’s disease.
I’m sorry did you say “a virus”? Injected directly into the brain?
Generally, gene therapy approaches have involved the use of viruses for delivering the new DNA. These genetically modified viruses have had all the disease causing component removed, allowing us to use the virus as an efficient biological delivery system. Viruses by their very nature are very good at infecting cells, so if we remove the disease causing components, what is left is a very effective courier.
Taking this approach one step further, we can take genes involved with dopamine synthesis and insert them into an empty virus. By then injecting this virus into the brain, we could produce dopamine in any infected cells (it’s slightly more complicated than that, but you get the basic idea).

Gene therapy for Parkinson’s disease. Source: Wiki.Epfl
The scientists at Oxford Biomedica used their virus to transfer three genes (aromatic amino acid dopa decarboxylase (AADC), tyrosine hydroxylase (TH), and GTP-cyclohydrolase 1 (GCH1)) into cells in a region of the brain called the striatum. These three genes are all critical in the production of a chemical called dopamine – the chemical that is lost in Parkinson’s disease.

GCH1, TH and AADC in dopamine production. Source: ScienceMag
The striatum is where dopamine producing neurons release most of their dopamine, but in Parkinson’s disease the dopamine neurons gradually die off, resulting in less dopamine. This reduction in dopamine levels is associated with the appearance of the motor features observed in Parkinson’s disease. The scientists at Oxford Biomedica were hoping to reprogram cells (that usually do not produce dopamine) in the striatum to start making dopamine, and that this reprogramming would result in increased levels of dopamine in the brain and less movement issues.
Preclinical research in rodent and primate models of Parkinson’s disease indicated very positive results using the Prosavin virus (Click here for more information on this), and the company moved towards testing the product in a clinical trial. The trial was completed in April 2012, and the results of the study were published in 2014:

Title: Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy forParkinson’s disease: a dose escalation, open-label, phase 1/2 trial.
Authors: Palfi S, Gurruchaga JM, Ralph GS, Lepetit H, Lavisse S, Buttery PC, Watts C, Miskin J, Kelleher M, Deeley S, Iwamuro H, Lefaucheur JP, Thiriez C, Fenelon G, Lucas C, Brugières P, Gabriel I, Abhay K, Drouot X, Tani N, Kas A, Ghaleh B, Le Corvoisier P, Dolphin P, Breen DP, Mason S, Guzman NV, Mazarakis ND, Radcliffe PA, Harrop R, Kingsman SM, Rascol O, Naylor S, Barker RA, Hantraye P, Remy P, Cesaro P, Mitrophanous KA.
Journal: Lancet. 2014 Mar 29;383(9923):1138-46.
PMID: 24412048
The clinical study was an open-label trial conducted over 12 months at two research centres (France and UK). It was designed to assess the safety and efficacy of ProSavin after the virus was injected into the striatum on both sides of the brain in 15 people with advanced Parkinson’s disease. Both research centres were registered as separate trials at ClinicalTrials.gov (NCT00627588and NCT01856439).
ProSavin was found to be safe and well tolerated in the study participants. No serious adverse effects related to the virus (or surgical procedure) were reported, and significant improvements in the motor issues associated with Parkinson’s disease were found (the average Unified Parkinson’s Disease Rating Scale (UPDRS) score off medication in all of the patients at 12 months had improved from 38 at the start of the study to 27 (p=0·0001).
This video provides an explanation of the first clinical trial of Prosavin:
Following the completion of the clinical study, Oxford Biomedica decided to move ahead with a more potent virus, known as OXB-102. Preclinical testing indicates that OXB-102 is at least five-fold more potent than ProSavin (based on behavioural and movement analysis). No clinical trial of OXB-102 has started yet, but the company is currently seeking regulatory approval for a multi-centre phase I/II clinical trial to be conducted in Cambridge and London, UK and Paris, France in 2018 (we’ll have more information about this in the new year hopefully).
This sounds really fantastic. What’s could possibly be wrong with it?
Well, Prosavin is one of the small success stories in the gene therapy world.
And please remember that thus far there has only been an open-label trial of the Prosavin product, it really needs to be tested in a double blind study. In this first study, there was very little difference between brain scans of dopamine processing before the study started and 6 months into the study – this may be one of the reasons why the company is seeking a more potent virus.

Brain imaging from before treatment (B) and 6 months after (C). Red indicates dopamine processing in the striatum (Putamen).Source: TheLancet
Generally speaking, the gene therapy approach has demonstrated amazing results in preclinical studies in the lab, but the transition to the clinic has not been easy (click here for a good review of the field).
The first clinical attempt at gene therapy for Parkinson’s disease involved injecting a virus containing a gene called glutamic acid decarboxylase (GAD), which is an enzyme involved in the production of a chemical called GABA. The virus was injected into an area of the brain called the subthalamic nucleus, which becomes over-active in Parkinson’s disease. This brain structure is a commonly targeted site for deep brain stimulation operations. By introducing GAD production in the subthalamic nucleus, researchers were able to reduce the level of activity, but the clinical trials for GAD only produced modest results. The virus was well tolerated, but the clinical effect was limited.
Another clinical trial attempted to cause cells in the striatum to produce a chemical called neurturin (which is very similar to GDNF – we have previously written about GDNF, click here to read that post). The goal of the study was to prove neuroprotection and regeneration to the remaining dopamine neurons, by releasing neurturin in the putamen. Subjects were injected in the putamen with the virus and then the participants were followed for 15 months. Unfortunately, this study failed to demonstrate any meaningful improvement in subjects with Parkinson’s disease.
I see. So what is the new research that could help this situation?
This is Dr Viviana Gradinaru

Dr Viviana Gradinaru. Source: Breakthough
She is an Assistant Professor of Biology and Biological Engineering at Caltech. In addition to exploring various aspects of brain functioning in both normal and disease state conditions, her lab produces new tools for studying the brain.
She is particularly interested in using viruses to deliver genes into the brain. But please understand that she has no interest in the whole brain surgery approach. No sir, she wants viruses that can be injected in the arm (or consumed orally as a solution) and can then travel to the brain and infect specific groups of neurons.
Source: ScienceDirect
No expensive, invasive surgery.
Just targeted gene delivery in specific cells in the brain using carefully designed viruses.
Cool idea huh?
Can she do it?
Well, her research group is certainly having some success in achieving this goal:

Title: Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain
Authors: Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu WL, Yang B, Huber N, Pasca SP, Gradinaru V.
Journal: Nat Biotechnol. 2016 Feb;34(2):204-9.
PMID: 26829320 (This article is OPEN ACCESS if you would like to read it)
In this study, Dr Gradinaru and her colleagues began by engineering a “library” (or pool) of many different Adeno-associated viruses (AAV).
What are Adeno-associated viruses?
Adeno-associated viruses are a kind of virus that are popular with researchers because A.) they readily infect human and primate cells, and B.) they produce little (if any) immune response, and C.) they are non-pathogenic (don’t cause disease). Given these characteristics, AAVs have been used in most of the gene therapy clinical trials thus far:

AAV-based gene therapy clinical trials. Source: Wikipedia
They were originally discovered in the preparation of another type of virus, called an adenovirus (hence the name ‘Adeno-associated’). They were believed to simply be a contaminant of that preparation. Further research, however, revealed that AAVs belong to the Dependoparvo genus of viruses, which in turn belongs to the family Parvoviridae.
AAVs are single-stranded DNA viruses, and they are one of the smallest viruses (approximately 22 nm in diameter) with a non-enveloped capsid. The capsid is the shell surrounding the genetic material of the virus. Viruses are either enveloped or non-enveloped. “Enveloped” means that a second casing surrounds the capsid, providing further protection for the virus, while “non-enveloped” viruses have only the capsid.
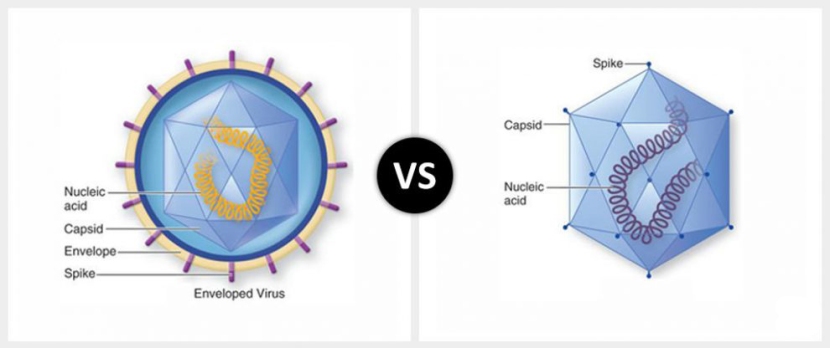
Enveloped (left) vs Non-enveloped (right) viruses. Source: Differencebtwn
Given the reduced amount of casing, non-enveloped viruses are generally more virulent (more infectious) than enveloped viruses (a good example of a non-enveloped virus is the influenza virus). Non-enveloped viruses do not survive outside of an organism for long though.

The AAV capsid. Source: Wikipedia
The AAV capsid (or outer shell) is composed of 60 proteins (as illustrated in the image above). There are at least 11 types of AAV (AAV2 being the most commonly used in research), and researchers can mix-and-match capsid proteins between the 11 different types of viruses to alter the infection characteristics of the viruses. Because the capsid is the first part of the virus to come into contact with cells, by making small changes to the proteins making up the capsid of a particular AAV, researchers can influence which kinds of cells the virus prefers to infect.
And this is exactly what Dr Gradinaru and her colleagues did in making their large pool of different AAV viruses. They randomly altered the capsid of each virus in a way that Dr Gradinaru and colleagues hoped would allow the virus to pass through the blood-brain-barrier (the protective membrane covering and protecting the brain).
They next injected these viruses into mice and one week later looked for AAV DNA in the brains of these mice. This analysis resulted in 13 AAVs that demonstrated robust ability to enter the brain and infect cells. One of these viruses in particular, AAV-PHP.B, represented approximately 25% of the viral DNA in the brains.
Next, in order to determine which cells the virus was infecting, the investigators needed a marker that would only be present in infected cells. They inserted a piece of DNA inside the AAV-PHP.B virus that would provide cells with the instructions to make a protein that glows when in the presence of ultraviolet light. That protein is called green fluorescent protein (or GFP).
Since it was discovered in Jellyfish in 1962, GFP has come to play an extremely significant role in research, allowing researchers to determine which cells are producing a particular protein (by replacing the DNA of that protein with the GFP DNA) or where in a cell a protein can be found (again by replacing the DNA of that protein with the GFP DNA). Its uses are many – so much so, that in 2008, the researchers who contributed to its discovery/utillity were awarded the Nobel prize in Chemistry (Click here to read more about this).
Organisms that produce GFP. Source: PNAS
By inserting GFP into the AAV-PHP.B virus, the researchers were able to determine which cells in each organ of the body was infected after the virus was injected into the tail of the mice. When the viruses infected a cell, that cell would turn green – providing the investigators with a rapid system of determining which cells are infected.

Comparing the viruses – green brains! Source: Nature
When they looked at the level of green fluorescent protein activation in the control virus (AAV9) treated brain compared to the AAV-PHP.B infected brains, they found that AAV-PHP.B exhibited an enormously higher level of infection (for example, 40-times higher in the cerebral cortex region of the brain and 92-times higher in the striatum).
And here is the really interesting result: The investigators found no significant differences in the levels of infection between the two viruses in the organs outside of the brain (such as the liver, heart, skeletal muscle and kidneys). The researchers had found a virus that seemed to have a strong preference for infecting brain cells.
And earlier this year (less than 18 months after the first research report was published) Dr Gradinaru and her colleagues published further results, demonstrating a refinement of this technology:

Title: Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems
Authors: Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sánchez-Guardado L, Lois C, Mazmanian SK, Deverman BE & Gradinaru V.
Journal: Nature Neuroscience. 2017; doi:10.1038/nn.4593
PMID: 28671695
In this research report, Dr Gradinaru and her colleagues published new findings based on two novel viruses, AAV-PHP.eB and AAV-PHP.S, which efficiently infect cells in the brain and spinal cord, respectively. Again the researchers put a green fluorescent protein in these viruses. Next, the researcher compared AAV-PHP.S with the control virus (AAV9), and they found that AAV-PHP.S very efficiently infected just the spinal cord while the AAV9 control virus infected very few cells in the brain (see the image below – the white line indicates the outline of the mouse brain as seen from above, with the spinal cord leading away to the left of each panel).

AAV-PHP.S virus compared with AAV9 virus. Source: Nature
To improve on this amazing technology, the researchers engineered the AAV-PHP.eB virus further so that the green fluorescent protein would only be activated in the cells that they wanted it to be activated in.
To demonstrate this, they designed a version of the AAV-PHP.eB virus that would only activate the green fluorescent protein in a region of the brain called the cerebellum (towards the back of the brain – see left panel on the image below). And taking this approach one step further, the investigators made sure that the green fluorescent protein was only activated within a particular type of cell in the cerebellum (those cells are called purkinje neurons). As the image below demonstrates, they could achieve this goal with impressive efficiency.

The cerebellum region of the mouse brain in green in the left panel, and only the Purkinje neurons have activated green fluorescent protein in the right panel. Source: Nature
Of particular interest to the Parkinson’s disease community, the researchers also demonstrated this remarkable cell-type specific approach with dopamine producing neurons (the cells that are badly affected in Parkinson’s disease).
Understand that most of the cells in the brain are infected with the AAV-PHP.eB, but the virus can be designed so that the genetic cargo that it is carrying is only activated in certain cells (such as dopamine neurons). This property opens up a lot of options for us when considering this technology for therapeutic purposes.
How could we use this technology in therapy?
Many ways.
For example, after being diagnosed with Parkinson’s disease, a virus containing the DNA instructions for making a beneficial chemical like GDNF could be delivered specifically to the cells that are affected in Parkinson’s disease, in an effort to protect those cells and keep them alive. In other words a neuroprotective approach to Parkinson’s disease.
Alternatively, before being diagnosed with Parkinson’s disease, you may become aware that you have a tiny genetic variation in every cell of your body that could make you susceptible to this condition. A virus targeting just brain cells could be injected into your body. That virus could contain the instructions for the correcting that genetic mutation, thus correcting most of the cells in your brain and limiting your chances of developing the disease. A gene editing approach to Parkinson’s disease.
Or in more advanced cases of Parkinson’s disease, perhaps a virus could be injected into the arm of a subject and that virus travels to the brain where it re-programmes astrocytes to become dopamine neurons – replacing the cells that have been lost in the condition (Click here to read a previous post about re-programming cells in the brain). This would be a cell replacement approach to Parkinson’s disease.
The options are basically limitless.
Have other research groups used this technology?
Yes. A lot of groups are now experimenting with it and some have already published results. One study in particular is of interest to us here, as it demonstrated a rescue of a mouse model of Parkinson’s.
Here is that study:
Title: AAV-PHP.B-Mediated Global-Scale Expression in the Mouse Nervous System Enables GBA1 Gene Therapy for Wide Protection from Synucleinopathy.
Authors: Morabito G, Giannelli SG, Ordazzo G, Bido S, Castoldi V, Indrigo M, Cabassi T, Cattaneo S, Luoni M, Cancellieri C, Sessa A, Bacigaluppi M, Taverna S, Leocani L, Lanciego JL, Broccoli V.
Journal: Molecular Therapy. 2017 Aug 10. pii: S1525-0016(17)30363-5.
PMID: 28882452
In this study, the investigators chose a genetically engineered mouse that produces too much of the Parkinson’s disease-associated protein, alpha synuclein. The particular type of alpha synuclein that these mice produce is a faulty version of the protein that is produced in people with a genetic mutation in the gene that provides the instructions for making the protein. At 6 months of age, these mice (called A53T-SCNA mice) gradually start accumulating alpha synuclein deposits throughout their brain, and by 10–12 months of age they start developing a loss of voluntary movements.
The researchers decided to try and rescue these mice by injecting them with an AAV-PHP.B virus that contained a piece of DNA that contains the GBA1 gene.
What is the GBA1 gene?
The GBA1 gene provides the instructions for making an enzyme, called Glucocerebrosidase. Approximately 5%–8% of people with Parkinson’s have a genetic mutation in the GBA1 gene (Click here and here to read more about this). This mutation causes a reduction in the activity of the Glucocerebrosidase enzyme.
What does Glucocerebrosidase do?
Glucocerebrosidase helps with the digestion and recycling of certain waste inside cells. The enzyme is located and active inside ‘lysosomes‘.
What are Lysosomes?
Lysosomes are small bags of digestive enzymes that can be found inside cells. They help to break down proteins that have either been brought into the cell or that have served their function and need to be digested and disposed of (or recycled).

How lysosomes work. Source: Prezi
Inside the lysosomes there are enzymes like glucocerebrosidase which help to break material down into useful basic parts. The lysosome will fuse with other small bags (called vacuole) that act as storage vessels of surplus material/waste inside a cell. The enzymes from the lysosome will mix with the material in the vacuole and digest it (or it break down into more manageable components).
Now people have two copies of each gene in their DNA, so if one doesn’t work properly there is a back up option. But if people have a genetic mutation in one of their GBA1 genes, cells in their body will often produce a normal version of the glucocerebrosidase enzyme and an abnormally short, non-functioning version of the glucocerebrosidase enzyme. In those cases the breaking down of waste inside the lysosome becomes less efficient. And if waste can not be disposed of or recycled properly, things start to go wrong in the cell.
Source: The Lancet
Generally, people with Parkinson’s disease and a GBA1 mutation will exhibit more severe symptoms than people without the GBA1 mutation (Click here to read more about this), but this is not always the case. In fact, there are cases of identical twins who both have a GBA1 mutation, but only one of them has Parkinson’s disease (Click here to read more about this). Regardless, increasing Glucocerebrosidase activity in individuals affected by this mutation represents a reasonable therapeutic approach to Parkinson’s disease.
And that is why the Italian researchers decided to have a look at whether an AAV-PHP.B virus that contained the GBA1 gene could rescue the alpha synuclein over-producing A53T-SCNA mice. So the question was: by increasing the level of waste recycling, could the investigator help these mice?
And the answer was: Yes.
Five-month-old A53T-SCNA mice were injected with with either the AAV-PHP.B GFP (control) virus or the AAV-PHP.B GBA1 GFP virus. Interestingly, glucocerebrosidase activity was strongly reduced in control A53TSCNA mice, suggesting that the build-up of alpha synuclein protein affects normal glucocerebrosidase protein processing.
The researchers found that the AAV-PHP.B GBA1 GFP virus significantly rescued of glucocerebrosidase enzyme levels and activity, which elicited a strong reduction of alpha synuclein in all of the brain regions examined (including the dopamine neurons – which are severely affected by Parkinson’s disease). The AAV-PHP.B GBA1 GFP virus injected mice also exhibited a strong recovery in learning and cognitive performance AND survived longer compared with the control treated mice.
Thus, a single injection of AAV-PHP.B was sufficient to rescue these mice, by infecting the majority of cells in the brain and only a fraction of cells in all the other organs. A rather remarkable achievement with an amazing new piece of technology.
So how did this technology “hit a rather significant obstacle” this week?
Source: NPR
The last step in the pre-clinical testing of novel medical treatments involves experimentation on our closest relatives in the animal kingdom: Non-human primates.
Whether you agree morally/ethically with this process, it is considered by the regulators in charge of our health care system to be a necessary step before experimenting in humans.
Researchers have been very keen to see if this new non-invasive approach could work in primates (with the obvious goal of testing this technology in humans). And the first research results involving primates were published last week… they were not very encouraging:
Title: Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain
Authors: Matsuzaki Y, Konno A, Mochizuki R, Shinohara Y, Nitta K, Okada Y, Hirai H.
Journal: Neurosci Lett. 2017 Nov 23. pii: S0304-3940(17)30958-8.
PMID: 29175632
In this study the researchers injected the AAV-PHP.B virus into the femoral vein of the leg of a young adult and an older adult primate (marmoset). They also injected age-matched control marmosets with standard AAV9 virus as a control measure. Both the AAV9 and AAV-PHP.B contained DNA for the production of green fluorescent protein, in order for the infected cells to be observed.
Six weeks after the viral injection, the brains (and other organs) of the animals were analysed to assess how wide spread the virus infection was. Unfortunately only a very limited infection (less than 3%) of neurons and astrocytes (the helper cells) was observed in both AAV-PHP.B and AAV9-treated marmosets. The researchers suggested that the results indicate species-specific differences in the ability of the viruses in their ability to get past the blood-brain-barrier (a protective membrane surrounding the brain). While the AAV-PHP.B and AAV9 viruses may be able to cross the mouse blood-brain-barrier with relative ease, the situation in the primate is different.
So it is back to the drawing board with this non-invasive, gene therapy approach for humans.
So what does it all mean?
The title of this post is a quote from the great Ben Bradlee.
Source: America.aljazeera
He was the executive editor of The Washington Post from 1968 to 1991. Critically, he was the man at the helm of the news paper during the Pentagon Papers challenge and he oversaw the publication of the stories documenting the Watergate scandal. In short, I think he would have made a very interesting dinner party guest (I’m currently reading Bernstein and Woodward’s ‘All the President’s Men‘). When Bradlee passed away in 2014, President Obama issued a statement saying “For Benjamin Bradlee, journalism was more than a profession — it was a public good vital to our democracy”. Many researchers feel the same way about their own profession (maybe not that it is ‘vital to our democracy’, but a public good nonetheless).
Hence, the quote ‘You never monkey with the truth’.
In scientific research, you definitely don’t always get the result you want and you should never monkey with the results. You present the result that you get. Period. In this post, research involving some monkeys has indicated that the road forward for non-invasive gene therapy is not going to be as easy as we had initially hoped.
But this does not spell the end of this line of research.
It simply means that alternative routes will be required as we move ahead. We will need to conduct new screening studies to identify viruses that will cross the blood-brain-barrier with greater efficiency (especially in primates). This will require a greater understanding of the differences between the mouse and primate blood-brain-barrier. In addition, further comparative analysis between mice and primates will be required for specific populations of cells (such as dopamine neurons) in order to determine what differences exist that could alter infection efficiency. Some of this data may already exist, but the identification of new engineered viruses may take some time.
I’m very keen on this particular areas of research and I will be keeping a close eye on it.
The banner for today’s post was sourced from Pixabay










Thanks, Simon! (You must write fast : )
LikeLike
Not fast enough 🙂
LikeLike
I am curious if the Japanese researchers who published this disappointing report could repeat the positive result shown by others using mice and the same virus cultures and technique they used in primates. I also wonder if the researchers who originally experimented with the mice had an opportunity to run the test in primates.
Simon, thank you for keeping this very informative forum.
LikeLike
Hi Felix,
Glad you like the site – what a great question! I probably should have mentioned it in the post that YES, the researchers confirmed the previous results in mice. Using a AAV-PHP.B virus that cause cells to produce high levels of green fluorescent protein (and AAV9 virus as a control), they intravenously injected 3-week-old mice and 4 weeks later found wide-spread presence of fluorescent green protein in the brains of the AAV-PHP.B injected mice (and very little in the AAV9 control brain), confirming previously published results.
Thanks for bringing this up. I will add a paragraph to the post, pointing this detail out.
Kind regards,
Simon
LikeLike