|
On this website, we regularly talk about a Parkinson’s-associated protein called Alpha Synuclein. It is widely considered to be ‘public enemy #1’ in the world of Parkinson’s research, or at the very least one of the major ‘trouble makers’. It is a curious little protein – one of the most abundant proteins in your brain. But did you know that there are different ‘species’ of alpha synuclein? And recently researchers in Florida announced that they had identified an all new species of alpha synuclein that they have called “P-alpha-syn-star” or Pα-syn*. In today’s post, we will discuss what is meant by the word ‘species’, look at the different species of alpha synuclein, and explore what this new species could mean for the Parkinson’s community. |
Source: Nationalgeographic
This microscopic creature is called Macrobiotus shonaicus.
Isn’t it cute?
The researchers that discovered it found it in a Japanese parking lot.
It is one of the newest species of life discovered to date (Click here for the research report). It is a species of Tardigrade (meaning “slow stepper”; also known as a water bear or moss piglet). And for the uninitiated: Tardigrade are remarkable creatures.
Tardigrade. Source: BBC
They measure just 0.5 mm (0.02 in) long, there are approximately 1,150 known species of them, and they have been around for a VERY long time – with fossil records dating back to the Cambrian period (500 million years ago).
The tree of life (try and find the dinosaurs). Source: Evogeneao
But most importantly, tardigrade are EXTREMELY resilient:
- they are the first known animals to survive in hard vacuum and UV radiation of outer space. Some of them can withstand extreme cold – down to temperatures of −458 °F (−272 °C), while other species of Tardigrade can withstand extremely hot temperatures – up to 300 °F (150 °C) (Click here to read more)
- they can withstand 1,000 times more radiation than other animals (Click here for more on that)
- some species of Tardigrade can also withstand pressure of 6,000 atmospheres (that is nearly SIX times the pressure of water in the deepest ocean trench – the Mariana trench! Click here for more on this)
- They are one of the few groups of species that are capable of suspending their metabolism; surviving for more than 30 years at −20 °C (−4 °F – Click here to read about this)
They are utterly remarkable creatures.
Great, but what does this have to do with Parkinson’s?
Nothing.
But if we are going to talk about new ‘species’ in today’s post, I just thought they would make a nice place to start.
I see. Shall we move on?
That would be a good idea.
When people consider of the word ‘species’, they usually think of the standard dictionary definition: a group of living organisms consisting of similar individuals capable of exchanging genes or interbreeding. Think: birds, bees, us.
But as you can see the dictionary has two definitions for the word ‘species’, and it is the second definition that is regularly used in biology when we talk about proteins.
What is a protein?
Proteins are large biological molecules, consisting of one or more long chains of amino acids.
They are the building blocks upon which all of us exist; they are an essential part of every living organisms. Derived from DNA (via a messenger molecule called RNA), they are created in a carefully controlled, multi-component process that is occurring continuously in our cells.
How are proteins made?
Ok, so you remember your high school science class when the adult at the front of the class was explaining biology 101? Like me, you may have slept through it, but hopefully you will remember a little something about the teachers saying that Deoxyribonucleic acid (or DNA) gives rise to Ribonucleic acid (or RNA), and RNA gives rise to protein. Yes?
This is the central dogma of biology. Do you remember it?

The basic of biology. Source: Youtube
DNA provides the instructions or the designs for making a protein, RNA is the template (or a photocopy of the designs) for making a protein, and protein is… well, protein is generally considered the functional end product in the process (but it is slightly more complicated than that – we’ll come back to this is a moment).
RNA-producing regions of DNA are referred to as genes. And it is each of these genes that provide the instructions for keeping you alive. The process of DNA providing the RNA template is called transcription, and this occurs inside the nucleus of a cell – where the DNA is kept. The process of RNA being used to produce protein is called translation, and this occurs outside of the nucleus.
For a good (cute) beginner introduction of the translation process, watch this video:
Once a chain of amino acids has been generated via the instructions provided by a particular piece of RNA, the process of making a protein continues on through a series of steps called post-translational modifications. These changes will give rise to the functional protein that can actually ‘do stuff’.
The production of a protein. Source: lumenlearning
The critical part of the post-translational steps is protein folding.
Here is another video discussing protein folding:
Pretty complex huh?
But how are different species of protein made?
The process of making each protein is long and extremely complicated, and at every step something could go wrong or be altered. These changes result in a tiny alterations in the shape of the final protein. If the final version of that altered protein is left to roam around in its altered state, it would represent a particular ‘species’ of that specific protein.
Returning to the dictionary definition of ‘species’, the altered protein represents ‘a kind or sort’ or the protein.
A large number of protein species can arise from one single gene. And these different species of a protein can stem from any of the many steps in the life span of a protein.
For example:
1. Alterations in the gene itself – a genetic mutation can give rise to new species of a protein.
2. Changes in the expression of the gene – the level of activity of a gene can result in new species (including alternative splicing).
3. Disruptions in RNA processing – both during the production of RNA (transcription) and in the immediate aftermath, during the quality control checking steps, new species of a protein can arise.
4. Alterations in the translation of RNA – translation of RNA involves three steps: Initiation, Elongation, and Termination. There is also proteolytic processing, during which a protein might be cut and reduced in order to achieve additional functions. And during each of these steps, alterations can occur that results in different species.
5. Perturbation in the formation of complexes with other protein species – after the production of a protein is finished, it will often link up with other proteins (these groupings are called a ‘complex’), and any perturbations during this process can also generate different species
6. Disruption in the degradation of the protein – and finally, once a protein has done its job and is sent off to be recycled, the waste disposal system can fail which can result in a new species of protein (we shall very shortly see an example of this below).
(Source of this list)
During every step in the life-span of a protein, a new protein species can be formed. And this is actually rather remarkable. You see, millions of years of evolution has resulted in a very strictly controlled production line for each protein. Specific enzymes – with very defined activities and concentrations – regulate almost every phase in the conversion process.
And yet, at each step during the lifetime of a protein – from gene expression to protein degradation – a tiny perturbation can change the quantity or quality of a specific converting enzyme, which could result in a new protein species. Some of those species will be useless (and these are often removed quite rapidly), while others may actually have beneficial side effects.
But there is also the possibility of a protein species being nasty.
Is ‘nasty species’ what happens in Parkinson’s?
The short answer to this questions is:
Source: Wellbeing365
And the longer answer is: Maybe.
One of the best examples of this is a protein called Alpha Synuclein. It is one of the most common proteins in your brain (making up about 1% of the protein in each neuron). Genetic mutations in the gene (called SNCA) that provides the instructions for this protein can increase one’s risk of developing Parkinson’s. Plus, alpha synuclein protein is found to cluster (or aggregate) specific regions of the brain in people with Parkinson’s – particularly in circular structures called ‘Lewy bodies‘ (Click here for more on Lewy bodies).
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies. In the affected areas of the brain, aggregated alpha synuclein can be seen in the branches (or neurites) of cells – see the image below where alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.

Examples of Lewy neurites (indicated by arrows). Source: Wikimedia
So alpha synuclein aggregates in cells in Parkinson’s? But is it ‘nasty’?
This is where the idea of ‘species’ becomes important.
You see, alpha synuclein is normally referred as a ‘natively unfolded protein’, in that is does not have a defined structure. When alpha synuclein is by itself, it will look like this:
Alpha synuclein. Source: Wikipedia
Alone, alpha synuclein is considered a monomer, or a single molecule that will bind to other molecules to form an oligomer (a collection of a certain number of monomers in a specific structure). In Parkinson’s, alpha synuclein also aggregates to form what are called ‘fibrils’. These can all be considered different ‘species’ of alpha synuclein.

Microscopic images of Alpha Synuclein (AS) monomers, oligomers and fibrils. Source: Brain
And between these different species, there is evidence emerging that suggests the oligomer species of alpha synuclein could be the ‘nasty species’ in Parkinson’s. They lead to the generation of fibrils, but they may also cause damage by themselves.

Source: Nature
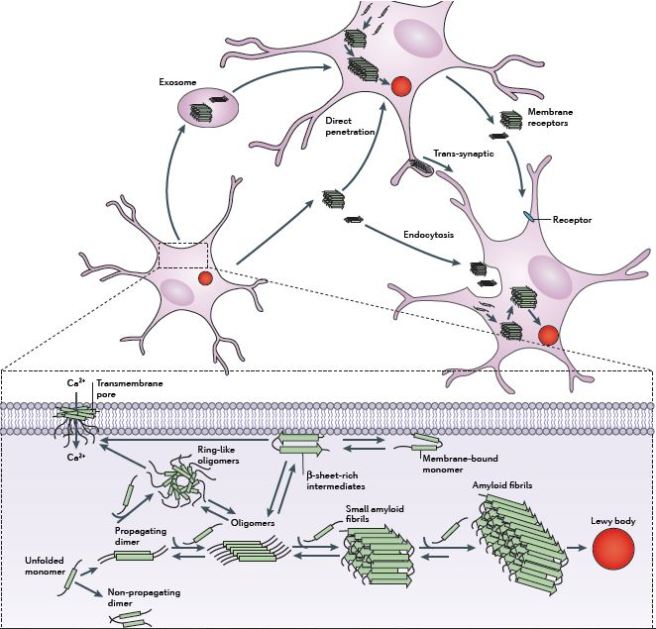
It is believed that the oligomer versions of alpha synuclein is being passed between cells – and this is how the condition may be progressing – and once inside the cell, these oligomer versions of alpha synuclein are causing other ‘natively unfolded protein’ monomer versions of alpha synuclein to become oligomers, which either bind with other oligomers to form fibrils or move on to another cell.
The passing of alpha synuclein between brain cells. Source: Nature
But very recently the idea of oligomer versions of alpha synuclein causing all the trouble has became more complicated with the publication of this study:
Title: Identification of a highly neurotoxic α-synuclein species inducing mitochondrial damage and mitophagy in Parkinson’s disease.
Authors: Grassi D, Howard S, Zhou M, Diaz-Perez N, Urban NT, Guerrero-Given D, Kamasawa N, Volpicelli-Daley LA, LoGrasso P, Lasmézas CI.
Journal: Proc Natl Acad Sci U S A. 2018 Feb 27.
PMID: 29487216
In this study, the researchers from the Scripps Research Institute in Jupiter (Florida) grew neurons from mice in cell culture. Five days later, they treated the cultures with either the fibril form of alpha synuclein or the monomer form of the protein (as a control). They then followed the cells for the next 14 days. During that time, the cells exposed to the fibril form of alpha synuclein began to display dense aggregates of the protein. Interestingly, the researchers observed two new species of alpha synuclein in these clusterings, which they call Pα-synF and Pα-syn* (pronounced “P-alpha-syn-STAR” which stands for alpha-synuclein truncated adamant and reactive).
The aggregates began to appear from about 2 days after the cells were exposed to the fibril form of alpha synuclein. The Pα-synF clusterings were wide spread, while the Pα-syn* species were localised in areas more densely packed with pα-synF. Pα-syn* was found to accumulate exclusively in neuronal cells. And the control cells which were exposed to alpha synuclein monomers instead of fibrils did not exhibit any accumulation of Pα-syn aggregates.
Source: PNAS
Next the investigators looked in the brains of mice that were exposed to the fibril form of alpha synuclein, and they found clusters of both Pα-synF and Pα-syn*. They also observed Pα-syn* in the postmortem brains of people who passed away with Parkinson’s. In these brains, Pα-syn* was typically found to be localised very close to the Lewy bodies.
In their next series of experiments, the researchers sought to determine where the new species of alpha synuclein were actually coming from. They found that that when a cell tries to dispose of alpha-synuclein fibrils (via a recycling programme called autophagy), sometimes the process is incomplete. And this incomplete degradation of the alpha-synuclein fibrils leads to the formation of Pα-syn*. They demonstrated this further by chemically inhibiting autophagy, which caused a reduction in the production of Pα-syn*. The investigators also reported that pharmacological activation of autophagy increased the production of pα-syn*.
Next the researchers wanted to know what Pα-syn* was doing in the cell, and they found that Pα-syn* was attracted to and targeting mitochondria.
What are mitochondria?
Mitochondria are the power stations of each cell. They help to keep the lights on. Without them, the party is over and the cell dies.
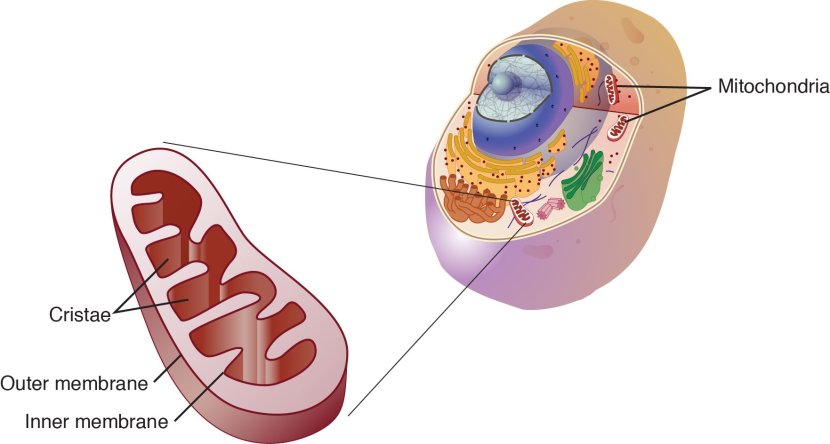
Mitochondria and their location in the cell. Source: NCBI
They convert nutrients from food into a chemical called Adenosine Triphosphate (or ATP). ATP is the fuel which cells run on. Given their critical role in energy supply, mitochondria are plentiful (some cells have thousands) and highly organised within the cell, being moved around to wherever they are needed.
Source: Mangomannutrition
The investigators found that not only did the Pα-syn* target the mitochondria, but that they were toxic for them as well. Once the pα-syn* attached to the mitochondria, the mitochondria would start to break down. Pα-syn* caused the mitochondria to undergo stress, fragment and die. Thus, not only did the researchers identify a new species of alpha synuclein, but they also concluded that their results implicate “pα-syn* as a key neurotoxic alpha synuclein species and a therapeutic target”.
The researchers in charge of the study: Prof. Corinne Lasmézas and Dr Diego Grassi. Source: Medicalxpress
Interesting. So why is this finding important?
This new finding could have important for several reasons. Most importantly, it could have major implications for ongoing clinical trials of ‘disease halting’ therapies.
Here at the SoPD, we have been very closely following the progress of two companies: an Austrian biotech called AFFiRiS:

Source: AFFiRiS
And a Californian company called Prothena:
Source: Prothena
Both companies have experimental treatments in clinical trials that specifically target alpha synuclein. As I mentioned above, it is believed that alpha synuclein is being passed from cell to cell. Both Prothena and AFFiRiS have developed treatments that capture and remove all of the alpha synuclein being passed between cells and this will hopefully halt – or at least slow down – the progress of Parkinson’s.
Prothena are the furtherest along in the clinical testing of their treatment with ongoing phase II studies of their drug called PRX002.
What is PRX002?
PRX002 is a monoclonal antibody. And before you ask: Antibodies are a critical part of our immune system. They are Y-shaped proteins which act as ‘alert flags’ for the immune system.

Monoclonal antibodies. Source: Astrazeneca
When a pathogen (an agent that causes disease or damage) is detected in your body, the immune system will quickly determine that it is not ‘self’ (meaning that it is not part of ‘you’ yourself). This judgement will be made by the identification of antigens on the surface of the pathogen. An antigen is defined as any substance or molecule that is capable of causing an immune response in an organism. If an molecule on the surface of the pathogen is not familiar to the immune system, it will be considered an antigen and an immune response will be initiated.
Antibodies bind to parts of the antigen called epitopes. Also known as antigenic determinants, an epitope is the part of an antigen that is recognised by an antibody. Antibodies by themselves can do a pretty good job of stopping pathogens, by blocking them from attaching to cells or by sticking together and clustering the antigens to prevent them from doing anything bad.

Antibody binding to antigens. Source: Venngage
The scientists at the biotech company Prothena have artificially engineered an antibody (PRX002) that binds to alpha synuclein (exactly which species or epitope of the protein it binds to is a company secret). PRX002 binds to alpha synuclein floating between cells and the immune system removes it. And this antibody has been injected into people with Parkinson’s and appears to be very effective at clearing alpha synuclein, we are now just waiting to see if this clearance results in a slowing of disease progression in Parkinson’s (Click here to learn more about PRX002).
AFFiRiS, on the other hand, is testing a vaccine which causes the body’s immune system to produce antibodies against alpha synuclein. In June 2017, AFFiRiS announced that the results of Phase I clinical study of their product AFFITOPE® PD03A. The company reported that the vaccine is causing an immune response (the immune system is generating antibodies against alpha synuclein) and that the vaccine was safe in people with Parkinson’s (Click here to read the press release, and click here to read more about the trial).
AFFiRiS has two vaccines for Parkinson’s that have been studied in phase I studies, and thus far 98 people with Parkinson’s or multiple system atrophy (MSA, a condition very similar to Parkinson’s – Click here to read a SoPD post on this topic) have participated in studies investigating either AFFITOPE® PD01A or PD03A. During these studies, participants were observed for up to 48 months (AFFITOPE® PD01A) or 12 months (AFFITOPE® PD03A), respectively. These observations have focused on long-term safety, immunological and clinical parameters, and the vaccine appears to be relatively safe.
Next, the company will be seeking to determine if they actually work. AFFiRiS is currently planning a Phase II efficacy trial, and we expect to learn more about that trial in 2018.
Vaccination. Source: WebMD
If Pα-syn* is the neurotoxic ‘bad guy’ in Parkinson’s, and it is the result of an incomplete degradation of alpha synuclein fibrils, then obviously any reduction in the levels of alpha synuclein floating between cells should have a positive effect on reducing the levels of Pα-syn*, and this should slow down the progress of the condition.
It would be even better if the antibodies being produced by the Prothena and AFFiRiS approaches are also targeting Pα-syn*. But maybe that is too much to hope for.
What does it all mean?
Researchers in Florida have identified a new species of the Parkinson’s-associated protein, alpha synuclein. This novel version of the protein can be found in postmortem sections of brain collected from people who passed away with Parkinson’s and it appears to target and have a toxic effect on the mitochondria (the power stations) in cells.
This result is encouraging as it provides further support for ongoing clinical trials that are targeting alpha synuclein protein which is floating between cells. Such treatment approaches could potentially halt the progression of the disease, by preventing alpha synuclein from being passed on.
It will be interesting to see where this new species research goes next, if it can be independently replicated, and if there are other yet to be discovered species of alpha synuclein.
A lot to look forward to here. Stay tuned.
EDITOR’S NOTE: The company Prothena which is mentioned in this post is a publicly traded company. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies mentioned on this page have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from Medicalxpress














Intriguing post, Simon! I expect I’ll need to read it several times to get it right in my head – back to the Amoeba Sisters first 🙂
Well done on explaining the un-explainable with humor so we can get it!
LikeLike
Hi Lisa,
Glad you like the post (and the Amoeba Sisters – they are great!). Let me know if you have any questions.
Kind regards,
Simon
LikeLiked by 1 person
@Simon
Another quality and thoughtful article Well done
Keith
LikeLike
Thanks Keith – glad you liked it.
Kind regards,
Simon
LikeLike
Simon,
Thank you! Excellent article as always,
But I find it unfortunate that researchers continue to ignore simple alternative therapies that are available today while they wait for the gold standard “double blind placebo controlled” silver bullet. Do you believe science will ever find a “one size fits all” solution to Parkinson’s? Would you agree that neurodegenerative disorders like Parkinson’s will most likely benefit from a “cocktail” of therapies to stop or slow progression, and that the earlier in life one begins to apply the combination of therapies the more likely one is to have success?
To wit, there are many simple “supplements” that have been studied in the lab both in cell cultures and in animal models that have been shown to aid in stopping protein mis-folding and enhance autophagy. Sadly “supplements” and “alternative therapies” continue to be dismissed by most researchers and all pharmaceutical companies.. Luckily Eastern medicine is not so philosophically opposed to the idea of supplements as potential therapeutics and so today’s patients are beginning to gain some “science” to back up their “alternative therapies”. Based on the ever increasing evidence that alpha-synuclein is an excellent target for treating Parkinson’s, why not advocate for trying simple “alternative therapies” that have some real science to explain why they hold potential while we wait for the expensive and elusive double blind placebo controlled trial “silver bullet”.
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0190160
http://www.jbc.org/content/282/8/5641.long
https://www.ncbi.nlm.nih.gov/pubmed/16893904
https://www.ncbi.nlm.nih.gov/pubmed/28267610
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4823968/
https://healthunlocked.com/ataxia-uk/posts/137505041/my-sca1-progression-has-stopped.-update-3-of-my-alternative-therapy-for-sca1.
Regardless, thank you again for the excellent article and links. I especially enjoyed the TED talk!
Joe in NY
LikeLike
Hi Joe,
Glad you liked the post – thanks for the interesting and thought provoking comment.
I am definitely in the “no silver bullet” camp. There are three components requires for a “cure” (1. Disease halting treatment, 2. Neuroprotective agent – to support remaining cells, and 3. Some form of cell replacement therapy). And sadly there is nothing on the horizon that does all three (eg. No disease halting therapy that brings back cells). Luckily, we have clinical trials for all three components though and this will hopefully result in a multi-pronged approach in the not too distant future.
I agree that sitting and waiting is not a great strategy, but one needs better methods of self assessment in order to know if these ‘cocktails’ are beneficial. I think there is a lot of useful compounds available (and my apologies for not responding to your previous comment where you outline your strategy – I will get to it), but the assessment is key. We really need a PD-DIY approach (pathetic teaser for a future post!).
Kind regards,
Simon
LikeLike
Oops. Nevermind. I was catching up on your articles starting with oldest to newest, and I just got to your most recent “Dilemma” article and see you already answered the question of a multi-pronged approach. Great article again 😀
LikeLike
Simon, This blog https://scienceofparkinsons.com/2017/12/03/lewy/
was interesting because there are basic unanswered questions about what ASN clumps do, this article begs the question, are they the cause of PD or a response to cell death in PD? These seem like really good questions (I know I am butchering your beautifully written concepts) but here is my question:
Does the discovery of Pa-SYN provide some of the missing info we have been looking for? Do you think this is possible?
LikeLike
Hi Pamela,
Thanks for your comment – glad you found that post interesting.
I personally tend to lean towards Lewy bodies being a cellular response to cellular stress, rather than actual cell death. There are genetic forms of PD that do not have Lewy bodies (some PARKIN variants for example).
As to whether pa-syn* is the missing piece of the puzzle, we shall have to wait and see. First, the result needs to independently replicated, but I’m sure there are a lot of people doing this already!
Kind regards,
Simon
LikeLike
Simon said:
“It would be even better if the antibodies being produced by the Prothena and AFFiRiS approaches are also targeting Pα-syn*. But maybe that is too much to hope for.”
That could be a mute point, if Pα-syn* is found to not exist outside of neurons.
From the research paper itself:
“Other intriguing questions are […] whether pα-syn* is propagated intercellularly. We did not detect pα-syn* in the culture medium, but it might have been due to low levels.”
LikeLike
Hi Jeffreyn,
Thanks for the comment and the great points.
If the ‘prion-like theory’ of alpha synuclein is correct, I think as long the immunotherapy approaches are sweeping up extracellular oligomeric/fribrillar alpha synuclein (which both companies say they are targeting), that should be enough to slow down the progression of the condition. If pa-syn* is solely an intracellular ‘degradation-gone-wrong’ phenomenon then limiting the amount of alpha synuclein being passed between cell should do the trick.
On the other hand, if pa-syn* is extracellular, then we have to hope that the immunotherapy approaches are also targeting it. Let’s see if anyone replicates these initial pa-syn* results first though, before we start to worry about such things.
Thanks again.
Kind regards,
Simon
LikeLike
Hi Simon,
Thanks for the substantial (and fast!) reply.
The research paper had another sentence that caught my attention, viz:
“These data show that any therapeutic approach aimed at accelerating the removal of fibrillary pα-syn aggregates/LBs with autophagy enhancers should be considered very carefully, as it might result in the production of more toxic and/or immunogenic pα-syn degradation products.”
This would seem to be something for the “Nilotinib for PD” trials to take note of.
LikeLike
Hi Jeffreyn,
Again, I would suggest we wait till the pa-syn* results have been replicated before speculating too much (there may also be other species of alpha syn). If validated, however, we could still lean on the immunotherapy approaches as a method of limiting the levels and spread of alpha synuclein oligomers/fibrils. In a hypothetical world, immunotherapy could be the front line treatment, and then boosting autophagy etc, could be secondary.
And if validated, I guess we will also need to develop better methods of detecting pa-syn* and other species in blood or cerebrospinal fluid, but as you suggested in your previous comment, the authors had a hard time doing this in culture solution, so this may be particularly tricky.
First things first: independent replication.
Kind regards,
Simon
LikeLike