|
Ursodeoxycholic acid (UDCA) has been proposed as a drug that could be repurposed for Parkinson’s. As a medication, it is called ‘Ursodiol‘ and it is used to treat gallstones. But there are absorption issues with UDCA: The passage of UDCA through the wall of the small intestine is slow and incomplete (Source). There may be a solution, however, called Tauroursodeoxycholic acid (TUDCA). Think of it as UDCA-2.0. It is more easily absorbed by the gut. And there is also good evidence to suggest that it has the same beneficial neuroprotective properties as UDCA. In today’s post we will discuss what exactly UDCA and TUDCA are, review the Parkinson’s research for both, and discuss why one of these drugs should be tested in the clinic for PD. |
Gallstones – ouch! Source: Healthline
Let me introduce you to your gallbladder:
It is one of the less appreciated organs; a pear-shaped, hollow organ located just under your liver and on the right side of your body. Its primary function is to store and concentrate your bile. Bile is a yellow-brown digestive enzyme – made and released by the liver – which helps with the digestion of fats in your small intestine (the duodenum).
Source: Mayoclinic
Now, let me introduce you to your gallstones:
Gallstones are hardened deposits that can form in your gallbladder. About 80% of gallstones are made of cholesterol. The remaining 20% of gallstones are made of calcium salts and bilirubin. Bilirubin is the yellow pigment in bile. When the body produces too much Bilirubin or cholesterol, gallstones can develop.
About 10-20% of the population have gallstones (Source), but the vast majority experience no symptoms and need no treatment.
Interesting intro, but what does any of this have to do with Parkinson’s?
One of the treatments for gallstones is called UDCA. And this compound is being considered for “repurposing” as a treatment for Parkinson’s.
What is UDCA?
UDCA = Ursodeoxycholic acid (also known as ursodiol).
Ursodeoxycholic acid/UDCA is a bile acid. It is a naturally occurring chemical that changes the composition of bile and helps to dissolve gallstones.
UDCA was first discovered in bears, hence the use of the root-word for ‘bear’ (urso-) in its name.
Bears – a source of potential PD treatments? Source: Constantinealexander
How does a a bile acid help with Parkinson’s?
Back in 1994, some researchers reported something very interesting about UDCA:
Title: Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group.
Authors: Poupon RE, Poupon R, Balkau B
Journal: N Engl J Med. 1994 May 12;330(19):1342-7.
PMID: 8152446 (This article is OPEN ACCESS if you would like to read it)
In this study the researchers were looking for a new therapy for primary biliary cirrhosis.
Primary biliary cirrhosis is a liver disease. It is an autoimmune condition which causes a slow, progressive destruction of the bile ducts of the liver, resulting in the build up of bile and other toxins in the liver. Given that ursodiol (UDCA) has is not toxic to liver cells in humans, the researcher hypothesised that long-term treatment with this drug might displace the build up of bile acids and thus reduce their toxicity in primary biliary cirrhosis. Crazier ideas have worked.
And guess what: It worked!
The investigators recruited and randomly assigned 145 patients with biopsy-proved primary biliary cirrhosis to receive either ursodiol (72 patients) or a placebo (73 patients) for 2 years. They found that long-term ursodiol therapy slowed the progression of the condition and reduced the need for liver transplantation.
Additional studies, however, found that this rescue effect may actually be due to cellular protective mechanisms rather than simply displacing the build up of other bile acids. Studies such as this one:

Title: A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation
Authors: Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ
Journal: J Clin Invest. 1998 Jun 15;101(12):2790-9.
PMID: 9637713 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers found that UDCA treatment in both hepatocytes and non-liver cells could block apoptosis (or programmed cell death). Apoptosis occurs when a cell is sick or damaged and it decides to shut down and die, initiating a programme of self destruction.
The investigators exposed liver cells to a range of apoptosis-inducing agents (from toxic doses of chemicals to apoptosis-causing proteins), and they found that co-administration of UDCA with each of these was associated with a 50-100% inhibition of apoptotic changes. They concluded that their “results suggest that UDCA plays a central role in modulating the apoptotic threshold” of the cells they tested.
But how does this apply to Parkinson’s?
More recently research groups have observed similar beneficial effects with UDCA in models of Parkinson’s:

Title: Ursodeoxycholic acid suppresses mitochondria-dependent programmed cell death induced by sodium nitroprusside in SH-SY5Y cells.
Authors: Chun HS, Low WC.
Journal: Toxicology. 2012 Feb 26;292(2-3):105-12.
PMID: 22178905
In this study, the researchers demonstrated that UDCA could protect dopamine cells grown in culture from apoptosis, and they also found that UDCA is doing this by regulating a specific cell survival pathway (PI3K-Akt/PKB).
And the neuroprotective effects of UDCA have also been found in a large screening study conducted by researchers at the University of Sheffield:

Title: Ursocholanic acid rescues mitochondrial function in common forms of familial Parkinson’s disease
Authors: Mortiboys H, Aasly J, Bandmann O.
Journal: Brain. 2013 Oct;136(Pt 10):3038-50.
PMID: 24000005
In this study, the investigators took 2000 drugs (including 1040 licensed drugs and 580 naturally occurring compounds) and conducted a massive screen to identify drugs that could rescue mitochondrial dysfunction in PARK2 (PARKIN) mutant cells.
Mitochondria are the power house of each cell. They keep the lights on. Without them, the lights go out and the cell dies.
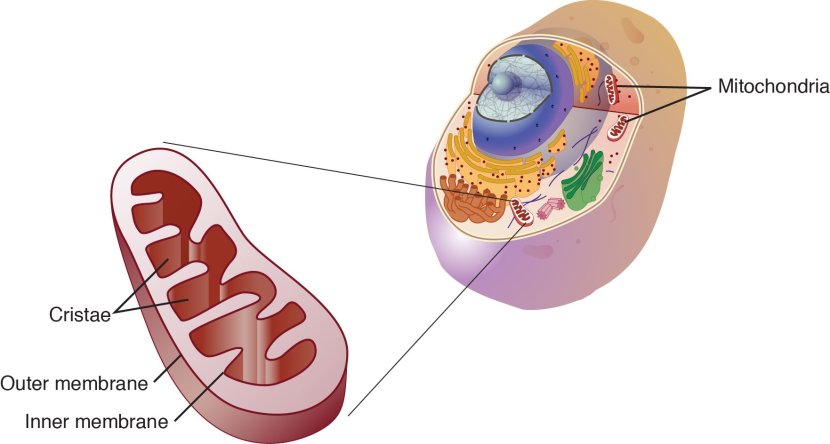
Mitochondria and their location in the cell. Source: NCBI
In certain genetic forms of Parkinson’s (such as those associated with mutations in the PARK2 gene), the mitochondria in cells becomes dysfunctional and may not be disposed of properly (Click here to read our previous post related to this).
In their huge screen of 2000 drugs, the researchers in Sheffield identified 15 drugs that could rescue the mitochondria dysfunction in the PARK2 skins cells. Of those 15 compounds, two were chosen for further functional analysis. They were:
- Ursocholanic acid
- Dehydro(11,12)ursolic acid lactone
(Did anyone else notice that both of these compounds have the root word for ‘bear’ in them? Coincidence? I think not)
Neither ursocholanic acid nor dehydro(11,12)ursolic acid lactone are FDA-licensed drugs. In fact, we have little if any information regarding their use in humans. Given this situation, the researchers turned their attention to a chemically related compound: UDCA.
UDCA. Source: Wikipedia
When the researchers tested UDCA on PARK2 skin cells, and they found that the drug rescued the mitochondrial function in those cells.
They next tested UDCA on skin cells from people with Parkinson’s who had mutations in the PARK8 (LRRK2) gene (G2019S; Click here for a previous post on this topic). The researchers had previously found impaired mitochondrial function and morphology in skin cells taken from people with PARK8 associated Parkinson’s (Click here to read more about this), and other groups had reported similar findings (Click here for more on this).
And when they treated the PARK8 cells with UDCA, guess what happened?
UDCA was able to rescue the mitochondrial effect in those cells as well!
Obviously these results excited the Sheffield scientists and they set up a collaboration with researchers at York University (UK) and from Trondheim (Norway), to look at the potential of UDCA in rescuing the fate of PARK8 flies. The results of that study were published in this report:

Title: UDCA exerts beneficial effect on mitochondrial dysfunction in Lrrk2 (G2019S) carriers and in vivo.
Authors: Mortiboys H, Furmston R, Bronstad G, Aasly J, Elliott C, Bandmann O.
Journal: Neurology. 2015 Sep 8;85(10):846-52.
PMID: 26253449 (This article is OPEN ACCESS if you would like to read it).
The researchers tested UDCA on flies (or drosophila) with specific PARK8/LRRK2 mutations (G2019S) display a progressive loss of photoreceptor cell function in their eyes. The mitochondria in the photoreceptor cells are swollen and disorganised. When the investigators treated the flies with UDCA, they found approximately 70% rescue of the photoreceptor cells function.
The researchers in Sheffield concluded that UDCA has a marked rescue effect on cells from a Parkinson’s-associated gene mutation model, and they proposed that “mitochondrial rescue agents may be a promising novel strategy for disease-modifying therapy in LRRK2-related PD, either given alone or in combination with LRRK2 kinase inhibitors” (Click here to read a previous post on the topic of LRRK2 inhibitors).
And this study was followed by another more recent report (from a different research group) which involved testing UDCA in animals:

Title: Ursodeoxycholic Acid Ameliorates Apoptotic Cascade in the Rotenone Model of Parkinson’s Disease: Modulation of Mitochondrial Perturbations.
Authors: Abdelkader NF, Safar MM, Salem HA.
Title: Mol Neurobiol. 2016 Mar;53(2):810-7.
PMID: 25502462
These researchers found UDCA rescued a rodent model of Parkinson’s (involving the neurotoxin rotenone). UDCA not only improved mitochondrial performance in the rats, but also demonstrated anti-inflammatory and anti-cell death properties.
Given all this research, the Parkinson’s research community have been very keen to test UDCA in clinical trials for Parkinson’s.
Is anyone testing UDCA in the clinic for Parkinson’s?
There is a Phase I clinical trial ongoing at the University of Minnesota – Clinical and Translational Science Institute.

This is a single group (20 participants) open label trial, in which each subject will receive daily UDCA treatment for six weeks.(Click here to read more about this). This trial will not be testing efficacy of the drug on Parkinson’s symptoms. It will focus on measuring UDCA levels in individuals, and determining the bioenergetic profile in those participants (as measured by brain imaging and blood tests). Basically, they want to see if UDCA is safe and active in people with Parkinson’s.
The study is expected to be completed in March, 2019 and we should have the results soon after that.
The Cure Parkinson’s Trust is also seeking to run a clinical trial for UDCA (Click here for more on this). The group are currently organising the funding for that trial.

Are there any side effects with UDCA use?
The side effects of UDCA/Ursodiol are relatively mild. Common side effects associated with Ursodiol use can include (Source):
- Diarrhoea or constipation
- Upset stomach, indigestion, or vomiting
- Dizziness
- Cough or sore throat
- Runny nose
- Back, muscle or joint pain
- Hair loss
- Dry skin or skin rash
- Headache
- Metallic taste in mouth
- Tiredness
But the real issue with UDCA is the ability of the gut to absorb it.
Following oral administration, the majority of ursodiol is absorbed by passive diffusion (a slow and inefficient process) and its absorption is incomplete – some of the drug journeys all the way along the gastrointestinal tract and is excreted. For the portion that is absorbed, ursodiol undergoes liver extraction to the extent of about 50% in the absence of liver disease.
Given this absorption issue, some researchers have turned their attention to an alternative version of UDCA, called TUDCA.
What is TUDCA?
TUDCA stands for Tauroursodeoxycholic acid.
Tauroursodeoxycholic acid. Source: Wikipedia
TUDCA is the taurine conjugate form of UDCA – this means that TUDCA is created when a molecule of the organic compound taurine is added to the UDCA structure (see image below).
The difference between UDCA (top) and TUDCA (bottom). Source: Anabolicminds
TUDCA is naturally produced, but in very small amounts.
Importantly, after oral administration, the TUDCA is absorbed by the gut via both active processes and passive diffusion. TUDCA appears to raise bile levels of UDCA more effectively than UDCA itself (Source & source). This is most likely due to the taurine molecules enhancing bioavailability – the process of binding a taurine molecule to UDCA is a ‘rate-limiting step’ (it slows down the absorption process), which could be avoided by simply starting with TUDCA.
But does TUDCA help in Parkinson’s?
No clinical evaluation of TUDCA in Parkinson’s has ever been conducted (to my knowledge), but there is pre-clinical data in models of Parkinson’s which suggests that TUDCA is as good as UDCA (if not better in some cases).
This research started in Portugal in 2012 with the publication of this report:

Title: Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson’s disease.
Authors: Castro-Caldas M, Carvalho AN, Rodrigues E, Henderson CJ, Wolf CR, Rodrigues CM, Gama MJ.
Journal: Mol Neurobiol. 2012 Oct;46(2):475-86.
PMID: 22773138
In this study, the researchers tested TUDCA treatment in a mouse model of Parkinson’s (using the neurotoxin MPTP). They found that UDCA had both neuroprotective (activation of the Akt pro-survival pathway) and anti-inflammatory effects, opening the door for TUDCA to be considered a modulator of neurodegenerative conditions like Parkinson’s.
These same researchers followed up that study recently with two further investigations:
Title: Nrf2 activation by tauroursodeoxycholic acid in experimental models of Parkinson’s disease.
Authors: Moreira S, Fonseca I, Nunes MJ, Rosa A, Lemos L, Rodrigues E, Carvalho AN, Outeiro TF, Rodrigues CMP, Gama MJ, Castro-Caldas M.
Journal: Exp Neurol. 2017 Sep;295:77-87.
PMID: 28552716
In this study, the researchers wanted to determine if TUDCA was having any effect on a protein called Nuclear Factor Erythroid 2-Related Factor 2 (or Nrf2). Nrf2 is an activator of various antioxidant and cytoprotective enzymes (Click here to read a previous post on Nrf2). The investigators found that TUDCA treatment increased the levels of Nrf2, as well as Nrf2 stabiliser protein DJ-1 and Nrf2 target antioxidant enzymes glutathione peroxidase and heme oxygenase-1. These results supported the idea that TUDCA has both neuroprotective and anti-oxidant properties. The investigators concluded that “TUDCA should be considered a promising therapeutic agent to be implemented in Parkinson’s”.
And they immediately followed up that study with this one:
Title: Novel insights into the antioxidant role of tauroursodeoxycholic acid in experimental models of Parkinson’s disease.
Authors: Rosa AI, Fonseca I, Nunes MJ, Moreira S, Rodrigues E, Carvalho AN, Rodrigues CMP, Gama MJ, Castro-Caldas M.
Journal: Biochim Biophys Acta. 2017 Sep;1863(9):2171-2181.
PMID: 28583715
In this study, the researchers replicated the previous study and supplemented it with cell culture experiments in order to better understand the mechanism by which TUDCA is having its beneficial effect. They found that TUDCA exerts its neuroprotective role in a PARKIN-dependent manner. PARKIN is a protein that is closely associated with Parkinson’s, and plays a key role in the removal of old/damaged mitochondria – thus helping the cell to remain healthy (Click here to read a previous post on PARKIN). After exposing cells in culture to the neurotoxin (MPTP) and treating them with TUDCA, the researchers noted a significant increase in the levels of PARKIN protein (see graph below).
Source: Sciencedirect
When the investigators reduced the levels of PARKIN in the cells, the neurotoxin and TUDCA treated cells failed to survive. The researchers concluded that their “results point to the pharmacological up-regulation of mitochondrial turnover by TUDCA as a novel neuroprotective mechanism of this molecule”.
And all of this research has given rise to the most recent study, which was published last week:

Title: Tauroursodeoxycholic Acid Improves Motor Symptoms in a Mouse Model of Parkinson’s Disease
Authors: Rosa AI, Duarte-Silva S, Silva-Fernandes A, Nunes MJ, Carvalho AN, Rodrigues E, Gama MJ, Rodrigues CMP, Maciel P, Castro-Caldas M.
Journal: Mol Neurobiol 2018 Apr 12.
PMID: 29651747
In this study, the researchers conducted an extremely extensive analysis of behaviour to better characterise the mouse model of Parkinson’s (MPTP) that they were using. And after this thorough investigation, they looked to see which aspect of movement they were rescuing with TUDCA treatment. They found that TUDCA administration – either before or after MPTP – significantly improved gait quality, swimming behaviour, and decreased foot dragging.
Importantly, when the investigators looked at the brains of these mice, they found that treating the mice with TUDCA either before or after the neurotoxin reduced levels of a protein called Paris (see graph below).
Source: Springer
Paris is a GTPase-activating protein, which are important regulators of signalling inside of cells. Increased levels of PARIS is observed in dying dopamine, and reducing levels of PARIS has been found to be neuroprotective (Click here to read more about Paris in Parkinson’s). There have been strenuous efforts to find inhibitors of PARIS (and these researchers may have just found one!).
The investigators concluded that the results of this study “should contribute to a subsequent clinical trial in humans and future validation of the therapeutic application of this bile acid in Parkinson’s”.
Has TUDCA ever been tested in the clinic?
As I mentioned above, TUDCA has not been clinically tested in Parkinson’s, but it has been recently trialled in Amyotrophic Lateral Sclerosis (ALS; also known as motor neurone disease and Lou Gehrig’s disease – Click here to read a previous post of this topic).
Here is the research report from that study:
Title: Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis.
Authors: Elia AE, Lalli S, Monsurrò MR, Sagnelli A, Taiello AC, Reggiori B, La Bella V, Tedeschi G, Albanese A.
Journal: Eur J Neurol. 2016 Jan;23(1):45-52.
PMID: 25664595 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers conducted a double‐blind, placebo-controlled clinical study on 34 people with ALS (being treated with riluzole) who were randomly assigned take either TUDCA or a placebo twice daily for 54 weeks.
The investigators found that TUDCA was well tolerated – there were no differences between the two groups for adverse events. And the proportion of responders (those who showed improvements) was higher in the TUDCA-treated group (87%) than in the placebo-treated group (this result is very similar to a previous 3 month clinical trial of UDCA in ALS – click here to read more about this). While the study suggests a positive trend regarding efficacy, it was too small to indicate whether TUDCA is demonstrating a statistically significant beneficial effect. The researchers concluded that the results justify a longer, larger trial of TUDCA in ALS.
Recently a Phase II trial started to assess the safety, efficacy and activity of AMX0035 – a treatment from Amylyx Pharmaceutical Corp which is a combination of Phenylbutyrate and TUDCA – for the treatment of ALS (Click here to learn more about this trial). This will be a randomised, double-blind, placebo-controlled study in which 132 individuals with ALS will be given either AMX0035 or a placebo twice daily for 24 weeks. The results of this trial will be available in mid-2019.
Here at the SoPD, we will be watching that trial very closely.
This year Johns Hopkins University has also initiated a Phase I/II trial of TUDCA in people with progressive Multiple Sclerosis (Click here to read more about this trial). The results of that trial will be available in mid-2020.
Are there any side effects associated with TUDCA?
This has never really been clarified. Having said that, usage of 500mg TUDCA daily for one year (in people after liver transplants) was not associated with any adverse effects (Source), which suggests that the compound is relatively safe. Diarrhea has been reported from dosages of 1,000- 1,500 mg of TUDCA (Source).
There is one potential concern regarding TUDCA: it increases glucose-induced insulin release (via the cAMP/PKA pathway), which in turn could increase insulin sensitivity (Click here to read more about this). This could affect people with diabetes or glucose intolerance – a common feature of Parkinson’s (Click here to read a previous SoPD post on this topic).
What does it all mean?
The bile acid Ursodeoxycholic acid (UDCA) and its derivative Tauroursodeoxycholic acid (TUDCA) have been found to be neuroprotective in models of Parkinson’s. These agents have also been found to have clinical benefits in certain conditions, making them very appealing drugs for testing in clinical trials of Parkinson’s.
While UDCA is approved by the Food and Drug Administration (FDA) for the treatment of primary biliary cirrhosis (the liver condition we mentioned near the top of this post), TUDCA is not approved. This means that TUDCA is not a drug that can be easily ‘repurposed’ (unlike UDCA). Therefore, the journey through the clinical trial process for use in Parkinson’s will be longer for TUDCA than it will be for UDCA.
Thus, until TUDCA is approved by regulators for another medical condition, it would be interesting to see concerted efforts to clinically test UDCA in Parkinson’s to determine whether this interesting compound has a future in the treatment of Parkinson’s.
ADDENDUM: 18/4/2018
So I have to be honest: the timing of this post was not coincidental.
I knew I would be speaking with Prof Oliver Bandmann the next day at the Parkinson’s UK Gretschen Amphlet Memorial lecture event here in Cambridge, and I was planning to ask him about the UDCA work (mentioned in this post) that they have been carrying out at the University of Sheffield (where he a Consultant Neurologist and a research group leader at the Sheffield Institute for Translational Neuroscience). And I was very pleased to hear him announce during his presentation that he has received funding for a clinical trial of UDCA in Parkinson’s (with support from the J P Moulton Foundation).
This is very good news, and we should see a press release about it soon.
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Wikipedia














Great article, but I found the last bit about FDA approval holding up use for PD confusing, since TUDCA is already being sold as a nutritional supplement by various companies.
LikeLike
Hi Lou,
Thanks for your comment – glad you liked the post.
Given that it is a widely available supplement, folks are free to use TUDCA if they so choose to, but their doctor’s hands are tired with regards to recommending it. Without clinical studies demonstrating that a.) TUDCA is safe for people with Parkinson’s, b.) it does not interact negatively with their medication, and c.) it has a beneficial effect, physicians will be reluctant to recommend it. A FDA approved trial process would greatly support its clinical use, if all of the above are addressed satisfactorily.
Kind regards,
Simon
LikeLike
Hi Simon,
Loved this post – very clear and informative.
Are you familiar with the website wehaveparkinsons.com and their product Restore Gold? It contains TUDCA along with other supplements that appear to address different aspects of Parkinson’s. I have been taking Restore Gold for 8 months, beginning a few months after I was diagnosed. My symptoms were mild, (and are, as I have not progressed). Physically, I have a tremor in my left hand and weakness in my left arm – that has not really changed (yet-I’m hoping it will improve with Restore Gold). But my anxiety, depression, insomnia all went away within a couple of months after starting Restore Gold. Also, my shoulder became less stiff. I also take Rasagiline, but I’m still unclear on what that is supposed to do? Anyway, there are many reviews on their website attesting to a regression of symptoms and a pausing of the disease. I am happy to see that there is much research being done in this area. However, the research process is so slow – I, for one, am not waiting. My doctor is, of course, skeptical, but said it will not be harmful. I remain optimistic.
Best regards,
Nadra
LikeLike
And while you’re at it, a spoonful of mannitol in your morning coffee isn’t a bad idea! But above all, try to stay cool! That mannitol might give you a bit of gas, but you’ll get over it after a while!
LikeLike
Any update on the TUDCA research?
LikeLike
Hi Simon,
Like Andrew, I’m also interested to know if there are any updates on TUDCA research. I know there was a trial in Sheffield, I think, but haven’t heard results. Have you heard anything?
Thanks,
Nadra
LikeLike
As far as I can tell, supply of TUDCA is a totally unregulated market. There is no way of knowing what is in a bottle of pills labelled TUDCA, unless of course you have access to the right equipment … https://en.wikipedia.org/wiki/Analytical_chemistry
Is anyone able to suggest a reliable source of TUDCA in the UK?
LikeLike
Hey Simon,
There is update on AMX0035. Take a look at the link below.
https://healthunlocked.com/cure-parkinsons/posts/149031941/what-are-your-thoughts-on-relyvrio-previously-known-as-amx0035-could-it-benefit-pd-patients-as-well
LikeLike