|
Recently researcher from the University of Cambridge reported that an imbalance in calcium and the Parkinson’s-associated protein alpha synuclein can cause the clustering of synaptic vesicles. What does this mean? And should we reduce our calcium intake as a result? In today’s post, we will review the research report, consider the biology behind the findings and how it could relate to Parkinson’s, and discuss what can or should be done. |
Me and Brie. Source: Wikipedia
When I turned 25, I realised that my body no longer accepted cheese.
This was a very serious problem.
You see, I still really liked cheese.
A bottle of red wine, a baguette and a chunk of brie – is there any better combination in life?
So obviously my body and I had a falling out. And yes, it got ugly. I wanted things to keep going the way they had always been, so I tried to make things interesting with new and exotic kinds of cheeses, which my body didn’t want to know about it. It rejected all of my efforts. And after a while, I gradually started resenting my body for not letting me be who I was.
We sought help. We tried interventions. But sadly, nothing worked.
And then things got really bad: My body decided that it didn’t have room in its life for yogurt, milk or even ice cream anymore (not even ice cream!!!). Basically no dairy what so ever.
There’s something’s missing in my life. Source: Morellisices
OMG. How did you survive without ice cream?
Well, I’ll tell ye – it’s been rough.
All silliness aside though, here is what I know: It is actually very common to develop a lactase deficiency as we get older – lactase being the enzyme responsible for the digestion of whole milk. In fact, about 65% of the global population has a reduced ability to digest lactose after infancy (Source: NIH). I am not lactose intolerant (one of the few tests that I actually aced in my life), but I do have trouble digesting a particular component of dairy products – which can result in discomfort and socially embarrassing situations (one day over a drink I’ll tell you the ‘cheese fondue story’). Curiously, that mystery ingredient is also present in products that have no dairy (such as mayonnaise – it absolutely kills me).
But spare me your tears, if one is forced to drop a particular food group, dairy is not too bad (if I am ever forced to give up wine, I swear I’ll go postal).
My biggest concern when I dropped dairy, however, was “where was I going to get my daily requirements of calcium?“.
Understand that calcium is really rather important.
Why is calcium important?
You will have grown up being told that calcium (from the Latin ‘calx’, meaning lime) is important for your bones, which is correct. But above and beyond that calcium is critical for the normal functioning of your entire nervous system.
Every nerve cell in your body is surrounded by a membrane – a skin that holds together the contents of the cell. This membrane also allows the cell to consume resources from the body, and it is critical to the cells ability to communicate with the other cells in the body.
A cell can communicate with its surroundings in several ways:
- Receptors on the outer surface of the cell membrane can receive chemical messengers
- The membrane can form a small bag and release chemical messengers
But for neurons in the brain, the membrane is key to one of the most important (and fastest) methods of communication, which is called an action potential.
What is an action potential?
An action potentials is an electrical impulse that is passed down the branch (or axon) of a neuron to the axon terminals at the tip of the neurons branches, where it stimulates the release of chemical messengers to pass the signal on to the next neuron.
Source: KhanAcademy
And the cell membrane is critical for a neuron’s ability to send this action potential.
Across the cell membrane, there is a ‘concentration gradient of ions’ (an ion is a molecule with a net electric charge due to the loss or gain of one or more electrons). This gradient simply means that there are more of certain chemical elements outside the cell than inside the cell (or vice versa). You can see this in the image below:
Source: Wikipedia
When a neuron is at rest, there is a high concentration of sodium (Na+) ions and chloride (Cl-) ions outside of the neuron (in the extracellular fluid) compared the situation inside the cell (the intracellular fluid) where there is a high concentration of potassium (K+) ions.
Source: Washington
During an action potential, as the impulse is moving along the axon (like a wave), this balance is reversed – sodium ions and chloride ions rush inside of the neuron, while potassium moves out. After the impulse has passed, there are active mechanisms that return the balance to normal (high concentration of sodium ions and chloride ions outside of the cell, etc).
This video will explain to you what an action potential is:
When the action potential reaches the axon terminals, it activates (voltage-dependent) calcium channels, which open up and allow calcium to come flowing into the terminal. Calcium is very important for the release of chemical messengers (like neurotransmitters) from the axon terminals. When calcium channels are blocked, neurotransmitter release is inhibited.
The critical thing to understand in the case of an action potential is that without calcium, communication between neurons becomes harder. And this is why calcium is so important in our diet.
Foods containing high levels of calcium. Source: Animalsaustralia
If nothing I’ve written here makes any sense, then hopefully this video will help:
Is there any research regarding the importance of calcium in Parkinson’s?
Yes. A great deal in fact.
You see, there is a grand theory of Parkinson’s based around calcium. It goes something like this:
Dopamine neurons (the cells that are particularly vulnerable in brain of a person with Parkinson’s) have a distinct property: They are autonomously active. That is to say, they are continuously generating a low frequency of activity, even in the absence of any direct input. This ‘pacemaker’-like activity is believed to be responsible for the sustained release of dopamine in the striatum (the caudate nucleus and putamen in the image below) which is considered to be necessary for proper functioning.
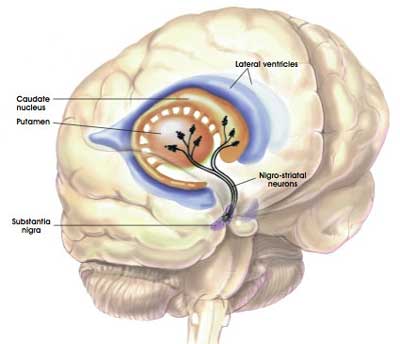
The projections of the dopamine neurons up into the striatum. Source: MyBrainNotes
This spontaneous electrical ‘pacemaker’ activity is believed to be dependent on calcium channels – particularly L-type calcium channels. If you block these calcium channels, the pacemaker property of dopamine neurons disappears.
A calcium channel. Source: Wikipedia
In the image above, there is a representation of a calcium channel embedded in the cell membrane. In the top left side of the image are three word written in red (Benzothiazepine, Phenylalkylamines & Dihydropyridines). These are inhibitors of calcium channels. They represent a class of medication called calcium channel blockers, which are used to treat hypertension (or long term high blood pressure).
Eight years ago, researchers noticed something interesting about calcium channel blockers that specifically target the L-type calcium channels:
Title: L-type calcium channel blockers and Parkinson disease in Denmark.
Authors: Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S.
Journal: Ann Neurol. 2010 May;67(5):600-6.
PMID: 20437557 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers collected the medical records of 1,931 people in Denmark who were diagnosed with Parkinson’s between 2001 and 2006. These records were age and sex matched to 9,651 records of healthy controls from the same register. After analysing all of the records, the investigators found that people prescribed with a type of medication called a L-type calcium channel blocker (dihydropyridines) were 27% less likely to develop Parkinson’s.
This finding supported a previous study that found a similar result (Click here to read more about that) and the same result was independently replicated a couple of years later (Click here to read that report).
And the result was also supported by postmortem analysis of the Parkinsonian brain:

Title: Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins.
Authors: Hurley MJ, Brandon B, Gentleman SM, Dexter DT.
Journal: Brain. 2013 Jul;136(Pt 7):2077-97.
PMID: 23771339 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers looked at where in the human brain the L-type calcium channels were present and in what concentration. They used sections of postmortem brain to carry out this analysis, looking at both healthy specimens as well as those from people who passed away with Parkinson’s.
In the normal brains, the distribution of the L-type calcium channels and certain calcium-binding proteins was not associated with regions of the brain that are prone to the selective neurodegeneration that is seen in Parkinson’s. But the investigators observed a very different picture in the Parkinsonian brains. Increased levels of L-type calcium channels and the calcium-binding proteins were found throughout the brains of people who passed away with Parkinson’s. Even in cases of early Parkinson’s (in people who passed away shortly after being diagnosed), increased levels of L-type calcium channels were even found in the cerebral cortex – a part of the brain largely unaffected in Parkinson’s.
These findings lead the researchers to conclude that “disturbed calcium homeostasis” may be “an early feature of Parkinson’s disease and not just a compensatory consequence to the neurodegenerative process”.
But how could calcium possibly be involved with the mechanisms underlying the neurodegeneration seen in Parkinson’s?
There are actually numerous ways that calcium could be playing a role – from mitochondrial function to oxidative stress. Click here to read a good short review on this topic.
In today’s post, we are going to be focusing on the interactions between calcium and the bad boy of Parkinson’s research: a protein called Alpha Synuclein.
Title: C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction.
Authors: Lautenschläger J, Stephens AD, Fusco G, Ströhl F, Curry N, Zacharopoulou M, Michel CH, Laine R, Nespovitaya N, Fantham M, Pinotsi D, Zago W, Fraser P, Tandon A, St George-Hyslop P, Rees E, Phillips JJ, De Simone A, Kaminski CF, Schierle GSK.
Journal: Nat Commun. 2018 Feb 19;9(1):712.
PMID: 29459792 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers wanted to look at how calcium could be affecting the normal functioning of the Parkinson’s-associated protein alpha synuclein.
What is alpha synuclein?
Alpha synuclein may sound like a distant galaxy, but it is one of the most common proteins in your brain – it makes up about 1% of the protein in each neuron. It is very plentiful.
Structurally, the protein is made up of 140 amino acids. And it looks like this:
Alpha synuclein. Source: Medicalxpress
Amino acids are the building blocks of biology. There are approximately 500 naturally occurring amino acids, but only 21 of them are actually involved with the instructions for making our bodies.
The 21 amino acids involved with making “us”. Source: Wikipedia
140 of these 21 amino acids line up to form the alpha synuclein protein, which can be subdivided into three major regions:
- the N terminus (amino acids 1–60 – sometimes referred to as the Amphipathic region)
- the non-amyloid-β component region (amino acid 61–95 – this is considered the aggregation-prone region)
- the C terminus (amino acid 96–140 – sometimes referred to as the Acidic tail)
And these region line up along the protein like this:
Alpha synuclein (with PD associated genetic variants indicated). Source: Mdpi
And what does alpha synuclein do?
This is a good question.
Like many proteins, alpha synuclein appears to have many roles, but something that is very clear is that it is certainly involved with functions in the synapse.
What is the synapse?
A synapse (from Greek synapsis meaning “connection or junction”) is a point on the membrane of a cell where communication is made with another neuron. Many synapses can be found at the tip of the branches (or axons) of a neuron, in the axon terminal region we were discussing above.
Source: Khanacademy
The synapse is where chemical messengers are parcelled into small bags called vesicles, which are then release into the extracellular space when a signal or action potential is sent down the axon. In the extracellular space, these chemical messengers can act receptors or channels on the surface of a neighbouring cell.
A synapse. Source: Sa2ir
Many years ago, researchers noticed that a lot of alpha synuclein protein can be found in the area of the synapse:
Title: Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal.
Authors: Maroteaux L, Campanelli JT, Scheller RH.
Journal: J Neurosci. 1988 Aug;8(8):2804-15.
PMID: 3411354 (This article is OPEN ACCESS if you would like to read it)
And this finding resulted in the idea that alpha synuclein may be involved with normal synaptic functioning.
More recently, we have learned that alpha synuclein is playing an important role in the handling of vesicles at the synapse. It acts as a protein buffer, helping to cluster vesicles at the synapse without affecting the release of the chemical messengers:
Title: Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2.
Authors: Diao J, Burré J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Südhof TC, Brunger AT.
Journal: Elife. 2013 Apr 30;2:e00592. doi: 10.7554/eLife.00592.
PMID: 23638301 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers found that Parkinson’s-associated genetic variants in the alpha synuclein protein (A30P) reduce the normal clustering activity of the alpha synuclein protein. The investigators also suggested that under certain stress or disease conditions, high levels of alpha synuclein could cause trouble as a result of severe synaptic vesicle aggregation, which would affect the normal release of the chemical messengers we were discussing above.
But what does alpha synuclein have to do with calcium?
Well, you may remember us discussing above that when an action potential reaches the axon terminals, it activates calcium channels, which open up and allow calcium to come pouring into the terminal. Calcium is very important for the release of vesicles from the synapse. Thus, like alpha synuclein, calcium is also found in very high concentrations in the synapse.
And many years ago it was discovered that calcium can bind directly to the alpha synuclein protein:
Title: Ca2+ binding to alpha-synuclein regulates ligand binding and oligomerization.
Authors: Nielsen MS, Vorum H, Lindersson E, Jensen PH.
Journal: J Biol Chem. 2001 Jun 22;276(25):22680-4.
PMID: 11312271 (This article is OPEN ACCESS if you would like to read it)
The researchers of this study found that not only did calcium bind to the novel C-terminal/acidic region (one of the three regions of the alpha synuclein protein mention above) of alpha synuclein, but this interaction caused changes to the function of the alpha synuclein protein. Amongst other features, calcium made the protein more susceptible to cluster (or aggregate) into the toxic version of the alpha synuclein that is believed to be involved in the pathology of Parkinson’s.
And this brings us back to the new research report mentioned above:
Title: C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction.
Authors: Lautenschläger J, Stephens AD, Fusco G, Ströhl F, Curry N, Zacharopoulou M, Michel CH, Laine R, Nespovitaya N, Fantham M, Pinotsi D, Zago W, Fraser P, Tandon A, St George-Hyslop P, Rees E, Phillips JJ, De Simone A, Kaminski CF, Schierle GSK.
Journal: Nat Commun. 2018 Feb 19;9(1):712.
PMID: 29459792 (This article is OPEN ACCESS if you would like to read it)
Of particular interest to the researchers in this study was the interactions between alpha synuclein and calcium, and how those interactions affect the normal functions of alpha synuclein.
Using a special technique (called ‘Chemical exchange saturation transfer-nuclear magnetic resonance‘ – please don’t ask me what that means), the researchers were able to watch what happens to the protein and calcium when they encounter each other. The investigators found that the alpha synuclein protein interacts with individual vesicles from the synapse using two regions: the N terminus and its C terminus.
When the researchers compared the binding of different ions to the C terminus, they found that calcium in particular had a particular affinity for alpha synuclein:
They also found that calcium increased the tendency of alpha synuclein to interact with vesicles. In fact, in the presence of calcium, alpha synuclein displayed specific clustering in the synapse, and the investigators showed that this could be reversed this effect by using a molecule that blocks calcium.
Another protein, called VAMP2 (which is also involved with synaptic function) was not affected by these calcium changes. Thus, the findings suggest that alpha synuclein is a calcium-dependent modulator of vesicles in the synapse.
Given that their results indicate that calcium mediates the interaction of alpha synuclein and vesicles, the researchers next asked whether this process could be involved with aspects of Parkinson’s. They tested this idea by treating alpha synuclein and vesicles with the chemical dopamine – the neurotransmitter that is severely depleted in the Parkinsonian brain.
The investigators found that the combination of calcium and dopamine promoted the clustering of alpha synuclein-attached vesicles. Interestingly, the researchers found that these dopamine-induced changes could be reversed back to normal by treating the cells with the calcium channel blocker, isradipine.
The researchers also noticed that the addition of dopamine to the combination of calcium and alpha synuclein appeared to cause some toxicity effect resulting in cell death in the experiment. So they next looked at whether the removal of calcium (using the calcium channel blocker isradipine) could reduce this cell loss.
And they found that it did:
The researchers concluded that these findings provide “a new view on the binding of alpha synuclein to synaptic vesicles, which might also affect our understanding of” Parkinson’s.
If nothing I have written makes any sense, the Parkinson’s UK medium blog has a very good write up on calcium and this research as well – Click here to read that post.
Are there any clinical trials targeting calcium?
Yes there are.
One in particular is being closely watched by a lot of people:
Source: STEADY-PD III
STEADY-PD III is a clinical trial for Parkinson’s that is investigating the utility of the calcium channel blocker that was used in the study above: ‘Isradipine’ (tradenames DynaCirc, Prescal).
Isradipine. Source: Dailymed
There are 3 classes of calcium channel blocker according to their chemical structure:
- Benzothiazepines
- Phenylalkylamines
- Dihydropyridines
Isradipine is a calcium channel blocker of the dihydropyridine class. It is prescribed for the treatment of high blood pressure in order to reduce the risk of stroke and heart attack. Prof Frank Church over at the ‘Journey with Parkinson’s’ blog has a great page on isradipine (Click here to read that post).
The double blind, Phase III STEADY-PD clinical trial is currently being conducted at 56 research centres across the US & Canada, enrolling over 300 people with newly diagnosed Parkinson’s to take either isradipine or placebo for 36 months (participants are randomly assigned to one of the two groups – click here to read more about the clinical study). The results of the trial should be available later next year (2019).
In addition to the isradipine study, there have also been clinical trials of a calcium channel blocking molecule called Apamin, which is a component of bee venom (Click here to read a previous SoPD post discussing the “bee venom” clinical trial).
Apamin. Source: Wikipedia
So what does it all mean?
Calcium is critical to our ability to function. It plays a fundamental role in our central nervous system.
There is a lot of evidence to suggest that it is somehow involved in Parkinson’s. From epidemiological research suggesting that people taking calcium channel blockers have a lower risk of developing Parkinson’s to postmortem analysis indicating changes in calcium channels in the brains of people with Parkinson’s.
Recently researchers have demonstrated that Parkinson’s-associated alpha synuclein also interacts with calcium and that this interaction may not be beneficial if levels of calcium get too high. The calcium channel blocker isradipine appears to correct the issues arising in this situation, however, which highlights a possible avenue for clinical intervention in PD.
Before you rush out and start loading yourself full of isradipine, understand that there can be side effects with this medication (Click here to read more about them) and also good reasons why this may NOT be the ideal drug for targeting L-type calcium channels in Parkinson’s (Click here to read more about that). But having said that, there are also efforts to look for better/more selective L-type calcium channel blockers as potential new therapeutics for Parkinson’s disease (Click here to read a research report on this topic).
While we will need to wait and see whether isradipine will be beneficial for PD, one thing we can be sure about is that we will be seeing a lot more research on calcium in the context of Parkinson’s.
So lay off the dairy and stay tuned.
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Nutraingredients
























nice post! Entertaining and beckoning me to learn some things – and even when I don’t follow completely your summary at the end makes it simple.
one question – is there supposed to be a not in this sentence? “…good reasons why this may be the ideal drug for targeting L-type calcium channels in Parkinson’s (Click here to read more about that)”
“However, in our study isradipine plasma concentrations approved for therapy were not neuroprotective in a PD mouse model. “
LikeLike
DKDC – I agree, reading the cited article leads me to believe that Simon intended to say “…good reasons why this may NOT be the ideal drug for targeting L-type calcium channels in Parkinson’s…”
LikeLike
Hi DKDC and Alex,
Glad you liked the post. And yes, you are both correct regarding the typo. Many thanks for pointing it out. It has been corrected now.
Kind regards,
Simon
LikeLike
Hi Simon
So you say at the end… lay off the dairy. So restricting dietary calcium might have a similar impact to blocking calcium channels?
Chris
LikeLike
Hi Chris,
Thanks for your comment. The simple answer to your question is I really don’t know, but I doubt it.
Understand that restricting levels of dietary calcium is not so much a bad idea (given how critical it is to various bodily functions), but rather a very difficult task. Food-wise, calcium is literally everywhere – a single kiwifruit, for example is considered low in calcium, but it still contains 20 milligrams of the stuff. And you are best to talk to your doctor before changing anything diet-wise (increases/decreases can impact the absorption/effect of medication).
The last line was a bit tongue-in-cheek, but there is some evidence suggesting that milk, ice cream, yogurt and cheese are associated with increased rates of disease progression (https://scienceofparkinsons.com/2017/09/18/food/), so maybe there is something to it. And research from the Honolulu Heart Study – a longitudinal study of “non-institutionalised men of Japanese ancestry, born 1900-1919, resident on the island of Oahu” – also supports the idea of lowering dairy intake. They found that calcium intake (from both dairy and nondairy sources) inferred NO increase/decrease in the risk of developing Parkinson’s. But they did find that the incidence of Parkinson’s increased with milk intake (drinking a large cup of milk per day doubled your chances – though the overall risk is still very low – https://scienceofparkinsons.com/2016/12/07/milk-yes-milk-and-parkinsons-disease/). Plus, dropping milk could mean less oxidative stress and inflammation biomarkers in your urine and blood according to a large Swedish study (https://www.bmj.com/content/349/bmj.g6015).
While I didn’t really answer your question, I hope this information helps.
Kind regards,
Simon
LikeLike
Thanks Simon. I might carry on with my small organic kefir habit… Weighing up gut microbes vs dairy risk.
Chris
LikeLike
Hi Simon
I just wonder how a study like this might be modified to take into account different phenotypes. What if only group 5 had a statistically significant benefit? Should they continue? And wouldn’t there need to be an explanation why only one group benefited? What if there’s no explanation? Just trying to comprehend the phenotype approach…
LikeLike
Interesting, SImon. I’ve recently muddled my way to a low-dairy diet and undoubtedly feel and perform better – when I can stick to it! Gluten, now – also an irritant at least?
LikeLike
Hi
I take Atacand ( candesartan cilexetil) for hypertension and other meds for raised cholesterol as well as Parkinsons meds.
Atacand as far as I know is not a calcium channel blocker. Should I be talking with my doctor about changing to a ccb?
LikeLike
Hi Paul,
Thanks for your message. Atacand is not a calcium channel blocker – it is a selective AT1 subtype angiotensin II receptor antagonist.
I can’t answer your question as I am not a clinician, just a research scientist, and I am not aware of your personal medical history. The best person to have this conversation with is your doctor.
Sorry I can’t be more help.
Kind regards,
Simon
LikeLike
Yes sorry Simon, that was too specific a question.
But generally is it correct to say some studies suggest that a reduction in calcium may be beneficial to slow the rate of parkinsons disease progression? As per (https://scienceofparkinsons.com/2017/09/18/food/)
And also what may be helpful to reduce calcium for a person with hypertension and parkinsons is by discussing with their doctor about pros and cons for their circumstances, of being on a ccb medication vs other types of anti hypertensive meds as a ccb may reduce calcium in an even greater manner than diet reduction alone. Is that correct?
Thanks Paul
LikeLike
Some of us are on BP meds despite taking sinemet and having pd both of which can lower BP. A ccb seems like a reasonable question to ask the Dr about. And the studies seem to show a clear “who knows” as to whether it’ll help. But it may not hurt? Why not try the “kitchen sink” approach as long as it is safe? (“Everything but the kitchen sink”)
LikeLike
Hi Dkdc,
Best to talk with your doctor about these sorts of matters. I too learn towards the kitchen sink approach, but in moderation. A multi-pronged approach is required for PD I suspect.
Kind regards,
Simon
LikeLike
Such a great article! I found it helpful in understanding the steady trial and I am anxious to see some results from this trial! Also helpful in understanding the role of alpha synuclein or ASN. On a different but related note I am curious what you think of trials like PASADENA which seeks to directly remove ASN clumps. My concern is that indiscriminate removal of ASN could have long term detrimental effects. What do you think of this? Meanwhile I am reading everything I can on new drugs being tested such as calcium channel blockers specifically “L” channels. I still don’t understand exactly how israpidine falls short in terms of “L” channels. Thank you for writing this article and if you have additional light you can shed here it would be so appreciated.
LikeLike
Hi Pamela,
Glad you liked the post – we are all anxious to see the results of the trials.
Regarding the PASADENA study, I have mentioned it recently in another post (https://scienceofparkinsons.com/2018/05/03/berserker/). It is important to understand that the approach the researchers are using in the PASADENA study is not ‘indiscriminate’ in its removal of alpha synuclein. PRX002 (the drug being tested in the PASADENA study) is an antibody-based treatment that targets a specific aggregated (or clustered) form of the alpha synuclein protein which is believed to be toxic. It does not affect normal (un-aggregated) alpha synuclein. Exactly what PRX002 is targeting (the precise epitope) is a trade secret for company running the trial: Prothena.
As to the long term consequences of this approach, it all depends on where you stand in the great ‘alpha synuclein’ debate. If you think that aggregated alpha synuclein is the bad guy in PD, then removing it is probably a good idea. If, however, you think that aggregated alpha synuclein is simply an innocent man getting caught in the wrong place at the wrong time, then maybe it’s not such a great idea to remove it. There is some evidence to suggest that the aggregated form of alpha synuclein may be a defensive mechanism inside the cell – playing a role in protecting cells from viruses (https://scienceofparkinsons.com/2017/04/07/hepatitis-parkinsons-goes-viral/). Only time will tell if the PASADENA approach will work. One concern I do have with the study is that the clearance of alpha synuclein alone may not be enough. Perhaps they also need a anti-inflammatory or neuroprotective approach to real slow/stop PD. Follow up studies will be required to address this. Interesting times for Parkinson’s research.
Kind regards,
Simon
LikeLike
Simon, this is very helpful, I am considering the PASADENA trial if they will accept me. It is difficult to weed through the risks. For some reason the calcium blockers seem safer.
LikeLike
What about supplementing with Magnesium–a natural calcium blocker?
LikeLike