|
This week Austrian biotech firm, AFFiRiS AG, made an announcement regarding their experimental immunotherapy/’vaccine’ approach for Parkinson’s. In their press release, the company provided the results of a long-term Phase I clinical trial testing the tolerability and safety of their treatment AFFITOPE® PD01A. The treatment was found to be safe and well-tolerated in people with Parkinson’s. But there was one sentence which was particularly intriguing in the press release regarding clinical symptoms. In today’s post, we will discuss what is meant by ‘immunotherapy’, outline what this particular clinical trial involved, review the results, and explore what this could mean for the Parkinson’s community. |
Source: uib
I have previously mentioned on this website that any ‘cure for Parkinson’s’ is going to require three components:
- A disease halting mechanism
- A neuroprotective agent
- Some form of cell replacement therapy
This week we got some interesting clinical news regarding the one of these components: A disease halting mechanism
Clinical trial results from Austria suggest that a new immunotherapy approach in people with Parkinson’s is both safe and well tolerated over long periods of time.
What is immunotherapy?
Immunotherapy is a method of boosting the body’s immune system to better fight a particular disease.
It involves utilising the immune system of your body, and artificially altering it to target a particular protein/disease-causing agent that is not usually recognised as a pathogen (a disease causing agent).
Immune cells attacking a cancer cell. Source: Lindau-nobel
It is potentially a very powerful method of treating a wide range of medical conditions, and the research on immunotherapy is particularly robust in the field of oncology (‘cancer’). Numerous methods of immunotherapy have been developed for cancer and are currently being tested in the clinic (Click here to read about the many clinical trials now under way).
Many approaches to immunotherapy against cancer. Source: Bloomberg
One of the most promising of these cancer-based immunotherapy approaches is called CART immunotherapy (or chimeric antigen receptor (CAR) T-cell immunotherapy).
This video explains how CART immunotherapy works:
Interesting, but how is immunotherapy being used against Parkinson’s?
One of the big theories about how Parkinson’s progresses involves the idea that a toxic form of the Parkinson’s-associated protein, alpha synuclein, could be being passed from cell to cell.
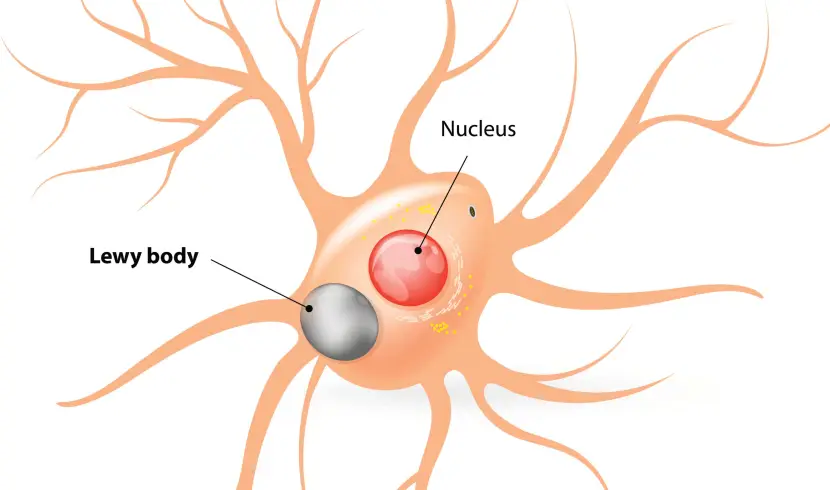
And as this toxic version of alpha synuclein is absorbed by each new healthy cell, it starts causing trouble in that healthy cell and this results in clustering (or aggregation) of protein, which is believed to lead to the appearance of Lewy bodies in those previously healthy cells.
Lewy bodies are dense circular clusters of alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies. In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells – see the image below where alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.

Examples of Lewy neurites (indicated by arrows). Source: Wikimedia
Is there any evidence of this transfer of the alpha synuclein protein?
So back in the 1990s, there were a series of clinical trials of cell transplantation conducted on people with Parkinson’s. The idea was to replace the cells that have been lost to the condition (Click here to read a previous post about cell transplantation). Many of the individuals who were transplanted have now passed away by natural causes and their brains have been examined post-mortem.
One very interesting finding from the analysis of those brains is that some of the cells in the transplants have Lewy bodies in them (up to 10% of transplanted cells in one case – Click here to read the research report on that case).
Above are photos of neurons from the post-mortem brains of people with Parkinson’s that received transplants. White arrows in the images above indicate lewy bodies inside transplanted cells. Source: The Lancet
This finding suggested to researchers that somehow this neurodegenerative condition is being passed on from the Parkinson’s affected brain to the healthy transplanted cells.
And researchers have proposed that the toxic form of the Parkinson’s-associated protein alpha synuclein may be the guilty party in this process (Click here to read more on this idea and the evidence for it).
So how is immunotherapy being applied to Parkinson’s?
One way of dealing with this problem of cell-to-cell transfer of the toxic form of alpha synuclein is to grab it as it is being passed between the cells, and remove it from the body.
This idea has given rise to a series of ongoing clinical trials that are using antibodies which target the toxic form of the alpha synuclein protein.
What are antibodies?
Antibodies are Y-shaped proteins that the immune system naturally and continuously produces to identify anything in the body that is ‘not self’ (that is, not a normally occurring part of you – think of viruses, bacteria, etc).

Monoclonal antibodies. Source: Astrazeneca
Antibodies act like alert flags for the immune system. When antibodies bind to something, they alert the immune system to investigate and potentially remove. Each antibody targets a very specific structure, while ignoring everything else.
In this fashion, antibodies are a very powerful method of removing items from the body that are causing trouble or not wanted.
And researchers have adapted this natural system for Parkinson’s using immunotherapy approaches. Currently, immunotherapy is being tested in Parkinson’s in two ways:
- Active immunisation – this approach involves the body’s immune system being encouraged to target the toxic form of alpha synuclein. The best example of this is a vaccine – a tiny fragment of the troublesome pathogen is injected into the body before the body is attacked, which helps to build up the immune systems resistance to the pathogen (thus preventing the disease from occurring).
- Passive immunisation – this approach involves researchers designing antibodies themselves that specifically target a pathogen (such as the toxic form of alpha synuclein, while leaving the normal version of the protein alone). These artificially generated antibodies can then be injected into the body.
Immunotherapy. Source: Acimmune
And which immunotherapy approach has this Austrian company taken?
AFFiRiS is clinically testing the ‘active immunisation’ approach for Parkinson’s.
Their experimental treatment involves a vaccine, which contains a tiny fragment of the toxic form of alpha synuclein (that has been synthetically engineered) being injected into people with Parkinson’s. This will hopefully build up an immune response against the toxic form of alpha synuclein, leading to the production of antibodies inside the body that will bind to and help remove the offending protein from the body.
Vaccination. Source: WebMD
Another biotech company, called Prothena, is attempting to target the toxic form of alpha synuclein using a passive immunisation (or ‘design your own antibodies’) approach to immunotherapy for Parkinson’s (Click here to read a previous SoPD post on that company).
And AFFiRiS and Prothena are not the only companies investigating the immunotherapy approach for Parkinson’s. There are other companies with early-stage programs in this area, including Biogen (BIIB054), AC Immune (ACI-870), Proclara (NPT088),NeuroPore (NPT200-11; in collaboration with UCB) and BioArctic Neuroscience (BAN0805; in collaboration with AbbVie).
And does the AFFiRiS vaccine actually work?
It depends on what you mean by the word ‘work’.
The data presented by the company thus far suggests that the vaccines being tested are definitely causing an immune response. So the vaccine is ‘working’ in that sense. The vaccines are also ‘working’ in that they are reducing the levels of alpha synuclein in blood and cerebrospinal fluid (the juice that your brain and spinal cord sits in).
But what we are not sure about is whether the vaccines are ‘working’ with regards to slowing down the progression of Parkinson’s.
We currently do not know if the vaccines are actually halting the condition.
…more on this below though.
What results have the company presented thus far?
So, it is important to understand that AFFiRiS has conducted numerous Phase I clinical studies for their vaccines for Parkinson’s.
They are the first researchers to bring a Parkinson’s vaccine to clinical testing in man and they are wisely being very cautious and prudent in their approach. They have also been very open with their results, providing regular updates and press releases with the end of each study.
In June 2017, the company announced that the results of their AFFiRiS011 study.
This was a Phase I clinical study of one of their vaccines, called AFFITOPE® PD03A. The company reported that the vaccine was causing an immune response (the immune system was generating antibodies against the toxic form of alpha synuclein) based on blood sample analysis, and that the vaccine was safe in people with Parkinson’s (Click here to read the press release, and click here to read more about the trial).
In that study, 36 people with early Parkinson’s (between 1.6-2.3 years since diagnosis) were randomly (and blindly) assigned either the AFFITOPE® PD03A high dose (75μg) vaccine, the AFFITOPE® PD03A low dose (15 μg) vaccine, or to a placebo treatment. The participants received 5 injections (4 for priming every 4 weeks and a 5th injection as a boost immunisation 9 months after the first injection). This study was conducted at two sites in Austria (Innsbruck and Vienna), and the participants were assessed over 52 weeks following their first injection.
I love Vienna. Source: Wien
Then on the 1st March 2018, the company announced results of another study called AFFiRiS009.
In this study, people with multiple system atrophy (or MSA, a condition very similar to Parkinson’s – Click here to read a SoPD post on this topic) were randomly (and blindly) assigned either the AFFITOPEs® PD01A vaccine, the PD03A vaccine, or a placebo treatment (Click here to read the press release and click here to read more about the trial). Again, both vaccines were well tolerated and found to be safe. This study was conducted at two sites in France (Bordeaux and Toulouse), and – again – the participants were assessed over 52 weeks following their first injection.
I’m a also big fan of Bordeaux (good wine). Source: France.fr
NOTE: One of the REALLY interesting results in this study was that while AFFITOPEs® PD01A produced an immune response in the MSA participants treated with it, there was no immune response to AFFITOPE® PD03A in MSA. That is to say, there was no significant differences in immune response in individuals treated with AFFITOPE® PD03A compared to those treated with placebo. We have previously discussed potential difference between MSA and Parkinson’s (Click here to read that SoPD post), perhaps this lack of response to AFFITOPE® PD03A in MSA highlights another difference.
Interesting, so what are the most recent results announced last week?
Last week the company provided the results of the AFF008 clinical studies (a series of four consecutive studies – Click here, here, here and here for the details of those studies). These results were initially presented at the Advances in Alzheimer and Parkinson’s Therapies (AAT-ADPD) Focus Meeting in Turino (Italy) earlier this year.
Source: Kenes
In these Michael J. Fox Foundation and Austria Wirtschaftsservice funded trial, a total of 32 people with early Parkinson’s (the average time since diagnosis was 2.6 years) were enrolled. 24 participants were randomly assigned to receive either:
- AFFITOPE® PD01A low dose (15 µg)
- AFFITOPE® PD01A high dose (75µg)
The remaining 8 people with Parkinson’s received standard medication/care – these individuals served as an observational comparison group. The participants received six injections: four for priming immunisations every four weeks, and then a 5th and 6th as “boost” and “re-boost” injection at 2 years and 3 years after the first injection. As with the previous trials, the goal of this Phase I trial was to determine safety and tolerability of AFFITOPE® PD01A, but for this particular study the researchers wanted to assess safety/tolerability over a long-term period.
In there announcement, AFFiRiS indicated that the vaccine was very well tolerated and no safety issues arose over the 4 year study. In addition, individuals treated with AFFITOPE® PD01A demonstrated a clear immune response (based on blood analysis). PD01-specific antibodies were also detectable in cerebrospinal fluid of individuals assessed.
Their results also indicate that AFFITOPE® PD01-induced antibodies preferentially bind to the toxic form of alpha synuclein (both oligomeric and fibrillar) compared to normal alpha synuclein (monomers) – Click here for a post that explains the differences), and the company suggested that there was a trend in reduction of oligomeric aSYN levels in blood as well as cerebrospinal fluid following treatment with PD01A (when assessed at week 26).
The company then stated that:
“Immunogenicity results after 4 years of treatment are encouraging and support the hypothesis that long-term disease management by targeting aSyn, a protein that is believed to contribute to the pathogenesis of Parkinson’s disease, with active immunotherapy seems to be feasible,”
It will be interesting to see the actually peer-reviewed published results of these studies.
So what does it all mean?
No, wait. We’re not summing up just yet.
We’re not finished.
We haven’t got to the really interesting part.
What do you mean???
The most interesting statement of the entire press release was this:
|
“Clinical scores for PD were stable during the entire study period, however, the study was not designed and not powered to evaluate clinical efficacy” |
Now, the big question is what was meant by “Clinical scores for PD were stable during the entire study period“???
Does this mean:
- People on AFFITOPE® PD01A experienced no clinical progression of Parkinson’s symptoms over the 4 years of the study???
- Or people on AFFITOPE® PD01A shared the same stable pattern of clinical scores as the control subjects???
I have reached out and contacted the company, asking for some clarification. They may not want to respond, which I will understand. But you have to admit that this is a rather remarkable statement to drop into a press release and not expand on further. And given that all of the participants involved in this trial were blind,… I am really intrigued.
I will let you know what they say in their response.
So what does it all mean?
An Austrian biotech is working on a vaccine for Parkinson’s. It targets the toxic version of the protein alpha synuclein. They have demonstrated across several Phase I safety clinical trials that their vaccine is working in that it elicits an immune response, but we are still waiting to see if this approach actually slows down the progression of Parkinson’s.
If successful, this approach would represent a major achievement in the battle against this condition – a method of slowing/halting the spread/progression of the condition – but it would also answer some important questions regarding the biology of Parkinson’s (such as “is alpha synuclein really the bad guy?”). And (as mentioned above) there are other companies taking immunotherapy approaches for Parkinson’s to the clinic. The company that is currently leading the pack is Prothena. They are currently recruiting participants for their Phase II trial which will be testing of their drug, PRX002 (also known as RG7935; Click here to read the research report on this study). That clinical trial is called the PASADENA study (Click here to learn more about the details of that trial).
Both companies (AFFiRiS and Prothena) are being careful in their approach to testing these first-in-man vaccines, and they are also well aware that immunotherapy has not yet been very successful for the other major neurodegenerative condition, Alzheimer’s. Several large (300+ participants) clinical trials of antibodies targeting the bad body of Alzheimer’s – a protein called beta amyloid – have failed to demonstrate any efficacy or slow the condition, which has led many researchers to question whether beta amyloid aggregation is the result and not the cause of the neurotoxicity (Click here for a good discussion on immunotherapy for Alzheimer’s).
Parkinson’s and Alzheimer’s are very different conditions though. And this week’s results from AFFiRiS are very encouraging. I will be looking forward to hearing about the start of a larger Phase II trial by AFFiRiS in the near future, and to the results of the Prothena Phase II trial which should be available in 2020.
EDITOR’S NOTE: Prothena is a publicly traded company, but the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. The company has not requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from AFFiRiS AG













Thanks for the great summation, Simon. I’ve been very interested to hear results on both of these efforts. Care to speculate on the likelihood that good news in the AFFiRiS approach (if indeed progression has been halted) also bodes well for the Prothena mono-clonal antibody approach? Any speculation on why Prothena never announced the (limited statistical) disease progression observations of their Phase 1 study?
One more thing I’ve been thinking about is your repeated observation that a cure consists of three things. Frankly, it seems to me that progression cessation and a dopaminergic replacement therapy alone should be quite sufficient. Why is the additional neuroprotective agent so essential in the presence of the other two?
Thanks once again! I’ll buy you a bottle of bubbly is you’ll share the good news with us!
LikeLike
Hi Tom,
Thanks for your comment – glad you liked the post.
I don’t like speculating on such things (particularly in situations where the clinical trial is ongoing and publicly traded companies are involved). I prefer to simply deal solely with the results. But I will say this: IF the immunotherapy approach against alpha synuclein does not demonstrate efficacy in its current form (that is, as a treatment in isolation), I would want to see it co-administered with other therapies before we subsequently give up on it.
My worry with the current beta amyloid immunotherapy clinical trials for Alzheimer’s that are failing at the moment, is that while clearing the protein may help, the other disease-related conditions (such as inflammation) that are left behind in the brain could be a reason why the Alzheimer’s continuous to progress. Thus, perhaps we need to rethink the way these treatments are being tested. Rather than in isolation, they could be combined with an anti-inflammatory approach and/or a neuroprotective agent. Just a thought.
Prothena didn’t say anything because Phase I trials are not designed to test efficacy. They are not statistically powered to do so. And any conclusions drawn from then could easily be found to be a false-positive/negative during the later Phase II/III trials.
And I think the ‘cure’ involving three components still stands. It largely depends on where you are in the course of the condition. If you have just been diagnosed, a disease halting treatment and dopamine replacement therapy may be enough. But if you are 5 – 10 years post diagnosis (and this will vary from person to person), I suspect a neuroprotective agent will also be required (see my comment above regarding the Alzheimer’s trials).
Thanks again for the interesting comment.
Kind regards,
Simon
LikeLike
Simon, Thanks as always for your careful scrutiny on what could be a break through trial. I am extremely curious about future Affiris trials in the US as timing is so important in the ability to get into these trials. The sooner they announce trials the better. However, this lack of clarification is worrisome. I *hope* that people on AFFITOPE® PD01A experienced no clinical progression of Parkinson’s symptoms over the 4 years of the study, but if that were the case, wouldn’t they be ringing in that news in every journal? Seems like it would be kind of a big deal. I, too, am ready to buy you a bottle of bubbly when we get some good news!
LikeLike
Hi Pamela,
Thanks for your comment – I hope all is well. As far as I’m aware AFFiRiS is yet to announce anything about their Phase II trial (happy to be corrected on this). It will be interesting to see how they plan to conduct it. I too am keen to see more information from these studies, but I am not pinning all my hopes on just this set of trials. There are lots of other clinical trial results coming our way soon. Several different therapeutic approaches to tackling various aspects of this condition (for example the Ambroxol results will hopefully be available before the end of this year). Stay tuned.
Kind regards,
Simon
LikeLike
Found this little tidbit recently:
“AFFiRiS vaccine trial has currently begun in humans [36]. Phase1 trial showed that it was (4 vaccine injections) safe, and 19 out of 22 participants developed antibodies against α-syn. In phase 2 trial over a 3 year observation, 8 out of 19 participants did not require increase in L-dopa therapy; and 5 out of 8 had no deterioration of the motor score.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5738184/
Not sure about the source…. My guess is this would fall under “stable”
LikeLike
Hi Double,
I hope all is well. Thanks for the comment and interesting link. That is a new one for me. I am not sure which Phase II trial they are talking about – as I mentioned in the comment above, AFFiRiS are yet to announce any plans for a Phase II. I might email the authors and ask for some clarification. I will let you know what they say.
Kind regards,
Simon
LikeLike
Simon, I’m pleased that I could share something you haven’t seen yet! Even if it’s innacurate. 🙂
I sure hope it is, because I could certainly go for no deterioration in the motor score. Even 5 of 22 sounds like a winner here.
LikeLike
Thanks, I really like the summary. Obviously, at this stage, not everything is clear a lot of experiments and analysis are missing, but nevertheless it is all in all very encouraging.
LikeLike
Hi Jens,
Thanks for your comment – glad you liked the post. Yes, it is encouraging, and the best part is that it is only one aspect of a multi-pronged approach towards attacking PD. There is so much other clinical work going on – it is hard to keep up!
Kind regards,
Simon
LikeLiked by 1 person
Simon,
Thanks for the thorough summary. Re: “Clinical scores for PD were stable during the entire study period, however, the study was not designed and not powered to evaluate clinical efficacy”. Just from a language perspective, it seems to me that this is an indication of efficacy in the results of this study. Otherwise, it would not be necessary to disclaim the design of the study as not for evaluating efficacy.
Bill
Philadelphia PA
LikeLike
Hi Bill,
It is a strange/curious way to word a press release. Fingers crossed it is as you say.
Kind regards,
Simon
LikeLike
Good point Bill. Why even mention scores at all if the weren’t trying to infer efficacy?
LikeLike