|
This week interesting research was published in the journal EMBO that looked at the Parkinson’s-associated protein Leucine-rich repeat kinase 2 (or LRRK2). In their study, the researchers discovered that lowering levels of LRRK2 protein (in cells and animals) affected the ability of Mycobacterium tuberculosis – the bacteria that causes Tuberculosis – to replicate. In today’s post, we will discuss what Tuberculosis is, how it relates to LRRK2 and Parkinson’s, and we will consider why this is potentially REALLY big news for Parkinson’s. |
Daedalus and Icarus. Source: Skytamer
In Greek Mythology, there is the tale of Daedalus and Icarus.
Daedalus was a really smart guy, who designed the labyrinth on Crete, which housed the Minotaur (the ‘part man, part bull’ beast). For all his hard work, however, Daedalus was shut up in a tower and held captive by King Minos to stop the knowledge of his Labyrinth from spreading to the general public.
Source: Clansofhonor
But a mere tower was never going to stop Daedalus, and he set about fabricating wings for himself and his young son Icarus (who was also a captive).
Being stuck in the tower limited Daedalus’ access to feathers for making those wings, except of course for the large birds of prey that circled the tower awaiting the demise of Daedalus and his son. But he devised a clever way of throwing stones at the birds in such a way, that he is able to strike one bird and then the ricochet would hit a second bird.
And thus, the phase ‘killing two birds with one stone’ was born (or so it is said – there is also a Chinese origin for the phrase – Source).
Interesting. And this relates to Parkinson’s how?!?
Well, this week researchers in the UK have discovered that a protein associated with Parkinson’s is apparently also associated with another condition: Tuberculosis. And they also found that treatments being designed to target this protein in Parkinson’s, could also be used to fight Tuberculosis.
Two birds, one stone.
What is Tuberculosis?
Tuberculosis (TB) is an infectious disease caused by the intracellular bacteria called Mycobacterium tuberculosis.
Not so cute. Source: Barnstablecountyhealth
Mycobacterium tuberculosis (or simply M. tuberculosis) is spread from person to person through tiny droplets in the air, that are released via coughing and sneezing. TB generally affects the lungs, but will often affect other parts of the body as the condition progresses.
Once breathed in, M. tuberculosis will either develop into latent TB (which does not cause illness and basically lays dormant in the lungs) or the full active disease that we know of as TB. About 10% of latent infections progress to active disease.
The active disease involves small cavities (or tubercles) developing in the lungs over time. Left untreated, this condition is progressive and fatal.
The damage TB does to the lungs. Source: ck12
The infection destroys lung tissue, causing infected individuals to start coughing. This results in M. tuberculosis being coughed up and spread through the air, where it can be inhaled by others.
At present, between 1/4 and 1/3 of the world’s population are infected with TB (Source: CDC), but most of those infected people do not have symptoms (latent TB). People with latent TB do not spread the disease.
- Coughing that lasts for three or more weeks.
- Coughing up blood.
- Chest pain or pain with breathing/coughing.
- Unintentional weight loss.
- Fatigue.
- Fever/night sweats.
- Chills.
The usual treatment for TB involves two antibiotics (isoniazid and rifampicin) being taken for six months. Two additional antibiotics (pyrazinamide and ethambutol) are also taken for the first two months of that six-month treatment period. And it is critical to continue taking the medication until the end of the treatment regime to avoid the development of drug-resistant strains of TB.
Is there an association between Tuberculosis and Parkinson’s?
This is a really interesting question.
In 1893, a research report was published suggesting an association between Parkinson’s and cell loss in the substantia nigra. It was the first time that the neurodegeneration in this region of the brain was highlighted in Parkinson’s. And we now know that this region is one of the most affected by the condition.
The report was written by Paul Blocq and Georges Marinesco:
Source: NCBI
An interesting detail of this report that is often overlooked by medical historians is that the brain came from a 38 year gentleman who was suffering from tuberculosis. He was admitted to the Charcot’s neurological ward at Salpêtrière (Paris – where both Blocq and Marinesco worked), because he also displayed signs of unilateral Parkinsonisms (rigidity and a left side tremor).
The individual subsequently passed away, and during the autopsy Blocq and Marinesco found a tubercle that had damaged the right substantia nigra (each side of the brain has a substantia nigra). This observation led Blocq and Marinesco to suggest that the Parkinsonism was most likely a complication of tuberculosis (Click here for an interesting article outlining the history of this report).
Yeah, it’s a very weak association, but more recently there has been a stronger association based on population data:
Title: Association Between Tuberculosis and Parkinson Disease: A Nationwide, Population-Based Cohort Study.
Authors: Shen CH, Chou CH, Liu FC, Lin TY, Huang WY, Wang YC, Kao CH.
Journal: Medicine (Baltimore). 2016 Feb;95(8):e2883. doi: 10.1097/MD.0000000000002883.
PMID: 26937925 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers conducted a nationwide, population-based, retrospective study investigating the risk of Parkinson’s in individuals diagnosed with TB. They used the enormous Taiwan National Health Insurance Database (which covered 99% of 23 million Taiwanese residents in 2000). The investigators collected data for individuals newly diagnosed with TB from 2000 to 2009, and matched each of those cases with 4 healthy, age/sex matched control cases. They excluded anyone who had Parkinson’s before or at the time of TB diagnosis from both groups.
The data from a total of 121,951 people with TB and 487,800 non-TB controls was used in this study. The researchers found that the TB-affected group were 1.4 times more likely to develop Parkinson’s at the first point in the analysis. But curiously as they followed the groups over time, this increased risk reduced:
Source: NCBI
The investigators speculated on why this reduction may have been observed. They questioned whether improvements in the early detection and treatment (with antibiotics like rifampicin) of TB over the period of analysis could have not only have treated TB but also prevented the “development of PD for subjects with active TB”.
And there is some research supporting the idea that antibiotics may have neuroprotective properties – we have previously discussed this on the SoPD website (Click here to read that post).
Interesting, but what does the new research published this week report?
This week, researchers from the UK published this report:
Title: LRRK2 is a negative regulator of Mycobacterium tuberculosis phagosome maturation in macrophages.
Authors: Härtlova A, Herbst S, Peltier J, Rodgers A, Bilkei-Gorzo O, Fearns A, Dill BD, Lee H, Flynn R, Cowley SA, Davies P, Lewis PA, Ganley IG, Martinez J, Alessi DR, Reith AD, Trost M, Gutierrez MG.
Journal: EMBO J. 2018 May 22. pii: e98694.
PMID: 29789389 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers wanted to investigate what role of Parkinson’s associated protein Leucine-rich repeat kinase 2 (or LRRK2) in macrophage cells.
What is LRRK2?
Also known as ‘Dardarin‘ (from the Basque word “dardara” which means “trembling”), LRRK2 is an enzyme that has many functions within a cell – from supporting efforts to move things around inside the cell to helping to keep the power on (involved with mitochondrial function).

The many jobs of LRRK2. Source: Researchgate
The gene that provides the instruction for making the LRRK2 enzyme resides on the 12th chromosome, in an area of DNA referred to as ‘PARK8’ (one of the Parkinson’s disease-associated genetic regions). The LRRK2 gene is located within the PARK8 region, and it is made up of many different sections, each of which is involved with the different functions of the eventual protein.
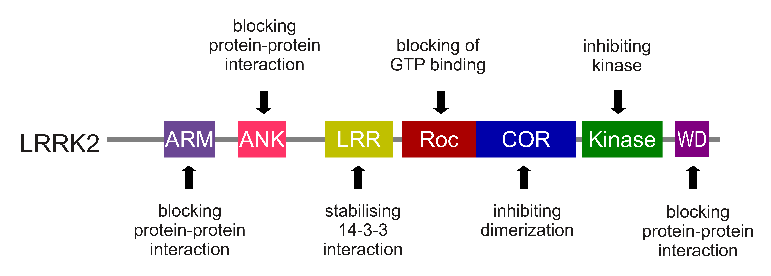
The regions and associated functions of the LRRK2 gene. Source: Intechopen
Genetic mutations within the LRRK2 gene are recognised as being some of the most common with regards to increasing ones risk of developing Parkinson’s disease (they are present in approximately 1-2% of all cases of Parkinson’s).

The structure of Lrrk2 and where various mutations lie. Source: Intech
As the image above suggests, mutations in the PARK8 gene are also associated with Crohn’s disease (Click here and here for more on this) – though that mutation is in a different location to those associated with Parkinson’s. And one particularly common Parkinson’s-associated LRRK2 mutation – called G2019S – is also associated with increased risk of certain types of cancer, especially for hormone-related cancer and breast cancer in women – Click here to read more about this. If you have a G2019S mutation, it is good to be aware of this association and have regular check ups.
The G2019S mutation (the name designates its location on the gene) is the most common LRRK2 mutation. In some populations of people it can be found in 40% of people with Parkinson’s (Click here to read more about this). But what is interesting about this mutation is that it gives rise to a LRRK2 enzyme that is hyperactive.
The structure of LRRK2 protein. Source: Wikipedia
As a protein, LRRK2 interacts with many different types of other proteins, and you can imagine that in a finely balanced environment like the cells that a mutant ‘hyperactive’ form of LRRK2 is going to cause problems. The consequences of this constantly active form of LRRK2 protein is believed to be the cause of cell death in LRRK2-associated Parkinson’s.
Ok, and what are macrophage cells?
Macrophage cells are blood cells that play an important role in the body’s response to injury or infection.
A macrophage seeking and reaching for pathogens. Source: Wikipedia
They are derived from monocytes, which are the ‘first responders’ of the immune system. Monocytes change into macrophage as they leave the blood system and enter damaged or affected area of the body.
They are very specialised cells involved in the detection, ‘phagocytosis‘ (the process of engulfing or consuming objects) and destruction of bacteria and other agents that are harmful to organism.
And if you are a pathogen, the last thing you want to see are macrophages approaching (because they are the last thing you will see). Macrophages are utterly ruthless. They consume anything that disagrees with them. And once consumed, the pathogen is broken down and spat out.
A macrophage. Source: Meducator
And how are macrophages associated with LRRK2?
Macrophage have very high levels of LRRK2. And this has led some researchers to question whether the immune system could be playing a role in LRRK2-associated Parkinson’s.
Ok, so what did the researchers find?
The researchers used TB as their pathogen (disease-causing agent) to test the response of macrophage cells response with and without LRRK2. To do this they engineered macrophage cells that do not produce any LRRK2. They then grew the cells and exposed them to M. tuberculosis (the bacteria that causes TB).
And guess what they found?
Macrophages with no LRRK2 were able to control M. tuberculosis replication significantly better than normal macrophages.
They also reported that treating normal macrophage cells with the LRRK2 kinase inhibitor GSK2578215A significantly restricted M. tuberculosis replication, further supporting the idea that inhibition of LRRK2 activity enhanced M. tuberculosis control by macrophages.
The researchers found that LRRK2 was having this function by blocking the formation of the phagosome.
What is the phagosome?
The phagosome is the bag that forms around a bacteria or pathogen as the macrophage is initially engulfing it. Hence the name ‘phagocytosis‘. Once the phagosome has fully matured, it binds to another bag inside the macrophage, called a lysosome, which is full of enzyme that help to degrade and break down the contents of the phagosome. The combination of the two bags is called a phagolysosome.
Phagosome. Source: Wikipedia
In this fashion, LRRK2 is basically blocking macrophages from doing their job: phagocytosis. The macrophages with no LRRK2 had no problems engulfing and destroying M. tuberculosis, while the normal macrophages had trouble consuming M. tuberculosis due to the actions of LRRK2.
And remember, in LRRK2-associated Parkinson’s (where there are genetic variations in the LRRK2 gene) we have a hyperactive form of LRRK2 protein, which would only make life more difficult for macrophages to do their job.
After investigating this phenomenon in cells, the researchers next turned their attention to mice. They exposed both normal mice and mice with no LRRK2 protein to M. tuberculosis and monitored them over 56 days. After just one week, the mice with no LRRK2 showed a significant reduction in levels of TB in their lungs. At 56 days after infection, the mice with no LRRK2 had significantly less number of lesions in their lungs.
One curious finding was that the absence of LRRK2 in macrohages alters the inflammatory response after M. tuberculosis infection. Inflammatory messengers (or cytokines) were significantly elevated in the mice with no LRRK2 protein (these cytokines incuded IL‐6, TNF‐α and IFN‐γ). This finding led the investigators to comment in their discussion of the results that the results demonstrate that “LRRK2 controls specific inflammatory pathways which need to be considered when evaluating the long‐term use of LRRK2 kinase inhibitors in PD patients“.
But equally, the researchers concluded that the “activity of LRRK2 can be a potential target for host‐directed therapies in tuberculosis“.
Is this the first time Parkinson’s genes have been associated with bacterial infection?
No.
A few years back, this report was published:
Title: The ubiquitin ligase parkin mediates resistance to intracellular pathogens.
Authors: Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, Schneider DS, Nakamura K, Shiloh MU, Cox JS.
Journal: Nature. 2013 Sep 26;501(7468):512-6.
PMID: 24005326 (This report is OPEN ACCESS if you would like to read it)
Genetic variation in the PARKIN gene have been associated Parkinson’s (Click here to read more about this). But genetic variation in the PARKIN gene have also been associated with increased susceptibility to intracellular bacterial pathogens, such as leprosy and typhoid (Source and Source). In this study, the researchers found high levels of PARKIN protein within lung tissue samples from people who had been infected with M. tuberculosis.
To investigate this further, the researchers conducted experiments that looked at what PARKIN was doing in cells infected by M. tuberculosis. They reported that PARKIN mediates the targeting of M. tuberculosis for disposal (via autophagy) and limits the replication of the bacteria. And they demonstrated this further when they infected normal mice and mice with no PARKIN protein. The investigators found that the mice with no PARKIN protein had a 10-fold increase in the levels of M. tuberculosis bacteria in some organs (21 days after infection).
The investigators repeated the experiments using flies and C. elegans (microscopic flatworms) that were both engineered to have no PARKIN protein. And they saw the same results – higher levels of M. tuberculosis in the organisms with no PARKIN – suggesting an “evolutionarily conserved” role for PARKIN in innate immunity (the immune system we are born with).
So what does it all mean?
UK researchers have identified a novel function for the Parkinson’s-associated protein LRRK2: it inhibits macrophage cells in the blood from consuming the pathogen bacteria that causes Tuberculosis.
The results are really exciting for two main reasons:
- They suggest that a new class of drugs being developed for Parkinson’s, may also be useful in TB. If this is the case, it means that LRRK2 inhibitor drugs will suddenly be on the radar screens of lots of big pharmaceutical companies. All of a sudden there may be a much larger market for this new class of drugs which will mean a lot more resources will be focused on them.
- The results also point towards a new theory of how Parkinson’s could be developing in certain individuals. If LRRK2 is preventing immune cells in the brain from degrading cell debris properly, leading to a build-up of rubbish – this could potentially cause inflammation and cell death.
On the first point, some readers may note the low incidence of LRRK2 genetic variants within the Parkinson’s community (only 1-2% of the folks with PD have a known LRRK2 mutation – Click here to read more about this). So how could LRRK2 inhibitors benefit everyone?
It is a fair question, but the absence of a ‘known’ LRRK2 genetic variant does not mean that a larger portion of the PD affected community would not benefit from using LRRK2 inhibitors. Perhaps lowering LRRK2 in individuals with normal levels of LRRK2 could also be beneficial in slowing down the progression of PD. Such an intervention may result from normal macrophage cells becoming even more efficient at clearing up debris and rubbish (I am simply speculating here).
More importantly, in the case of re-purposing some of these Parkinson’s LRRK2 inhibitor drugs for TB should be rather straightforward, particularly given that the blood-brain-barrier does not need to be addressed.
And on the second matter, such an idea brings into question whether bacterial pathogens could be playing a role in the development of Parkinson’s. It also suggests that we need to broaden our focus from thinking of Parkinson’s solely as a ‘neuro’ condition. As biochemist Dr Patrick Lewis from the University of Reading (one of the researchers involved in the study) commented: “The dogma in the Parkinson’s field has been to focus almost exclusively on what is happening to neurons in the brain to make them degenerate. This study reinforces why we should think more broadly about the events that cause neurodegeneration, and that some of the answers to Parkinson’s disease might come from immunology” (Source).
Overall, it is a very interesting result – one which (if independently replicated) will have major implications for both Parkinson’s and Tuberculosis.
Two birds, one stone!
The banner for today’s post was sourced from thefreshtoast













Ok. I need to educate myself. I found out today that 23andme thinks I have lrrk2 (I can’t even talk about basic info correctly!).
I need to reread past posts to see what this all means. Thanks.
LikeLike
Hi Dkdc,
Apologies for the delay in responding to your comment. The laptop died last week and with it all of the backroom components of the SoPD website. Replacing things has been chaotic.
If you do carry a LRRK2 variant, there are several important things to keep in mind.
1. Appreciate that we are still only chipping away at the iceberg with regards to our understanding of the genetics of PD. We know of a few regions of DNA (such as the LRRK2 gene) where errors in our basic biological coding can result in an increased risk of developing PD, but even these pieces of the puzzle are not clear. There is an elderly lady in Australia who has multiple genetic variants in the PARKIN gene. This genetic situation usually results in a very early onset of PD (in one’s 20s or 30s), and yet she is still fine in her 80s (and we have no idea why!).
2. Just because you may have a genetic variant in the LRRK2 gene does NOT mean that certain things are definitely going to happen. For a while now I have been drafting a post on the epigenetics of Parkinson’s. Epigenetics is all the stuff that determines who you are that isn’t written in your DNA. It is extremely complex stuff. Just the regulation of the activity of your DNA is a universe in itself.
Thus, it is very difficult to really conclude too much from the 23andMe test results.
That said, the results are still very useful information. They can be instrumental in getting an individual on to a particular clinical trial, if one is so inclined (personal choice entirely). But the results can also point you in the direction of things to keep an eye on (for example all of the LRRK2 inhibitor research).
I hope this helps. Have a look at the genetics page on the website (https://scienceofparkinsons.com/genetics-of-pd/) and I am happy to provide a better explanation of things via email if you wish.
Kind regards,
Simon
LikeLike
Thanks, IT does seem after reading a few things that having lLRRK2 and the variant I have doesn’t mean anything particular necessarily. Other than maybe a key to future treatment.
LikeLike
Ineresting. and well explained by Simon
LikeLike
Thanks! Glad you liked it… and that it made sense!
LikeLike
Hi Simon,
The question of whether bacterial pathogens could be playing a role in the development of Parkinson’s makes me wonder…
I was diagnosed with PD in 2009. In about 2001 I had picked up a large tick from the fields near our house. I developed a ring rash and was treated with (only) 5 days of antibiotics. About 3 or 4 years ago I had a blood test for Lyme but it wasn’t seen as conclusive. The interesting thing was the extremely low count of CD57 positive NK absolute Heparin. This didn’t mean a thing to me but after reading your article I looked it up and it seems to be related to an immune suppressant situation.
It’s a very long shot but I just wonder if there could be a connection… TB and Parkinson’s / Lyme and Parkinson’s? And an even longer shot (!) , could there also be in some cases a part played by genetic variation in the PARKIN gene via, as you say, increased susceptibility to bacterial pathogens?
I don’t know enough about this to know if there is anything in this but just in case…
LikeLike
Hi Mel,
Thanks for your comment and for sharing. I have long wondered about a viral/microbial influence in the development of Parkinson’s, so this research was of particular interest to me. With regards to Lyme, there have been cases of Parkinsonisms (PD-like features) appearing as a result of Lyme disease (for example: http://danielcameronmd.com/subacute-parkinsonism-complication-lyme-disease/), but as to whether there is a direct association I’m really not sure any evidence exists to support a link (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4622257/). I think we need to wait and see what research comes next on this topic (LRRK2 & PARKIN variants increasing susceptibility to pathogens), before we can really start to speculate too much.
Kind regards,
Simon
LikeLike
There are interesting links between Rab proteins, motors inside cells and the intake of pathogens. Links between Rab proteins and LRRK2 (and other PD-related proteins) are emerging all the time, so this is nice work
chris
LikeLike
Hi Chris,
Thanks for the comment. While I wonder about the specificity of some of the LRRK2 inhibitors used in the study, I really like this report. It provokes lots of questions and ideas (RABs included).
Kind regards,
Simon
LikeLike