|
The results of a recent clinical study for Parkinson’s conducted in Georgia (USA) has grabbed the attention of some readers. The study involved Niacin (also known as nicotinic acid), which is a naturally occurring organic dietary compound and a form of vitamin B3. The study was very small, but the researchers noticed something interesting in the blood of the participants: Niacin was apparently switching some of the immune cells from an inflammatory state to an anti-inflammatory state. In today’s post, we will discuss what Niacin is, how it relates to Parkinson’s, and we will consider some of the issues with having too much niacin in your diet. |
Source: Universal
It is one of the most common requests I get:
“Can you give an opinion on this supplement ____ or that vitamin ____ as a treatment for Parkinson’s?”
And I don’t like giving opinions, because (my standard disclosure) “I am not a clinician, just a research scientist. And even if i was a clinician, it would be unethical for me to comment as I am not familiar with each individual’s medical history. The best person to speak to is your personal doctor“.
But I also don’t like giving opinions because of a terrible fear that if I write anything remotely positive about anything remotely supplemental or vitamintal (is that a word?), a small portion of readers will rush off and gorge themselves on anything that sounds remotely similar to that supplement or vitamin.
So you will hopefully understand why I am hesitant to write this post.
But having said that, the recently published results of a small clinical study conducted in Augusta (Georgia, USA) are rather interesting.
The Augusta Masters. Source: Darkroom
When most people think of Augusta, they think of golf and the Masters Golf Tournament that is held there every year. I am of the Mark Twain mind-set when it comes to golf (“Golf is a good walk spoiled”).
Mr Samuel Langhorne Clemens. Source: Kenburns
But there is now something else that we can associate with Augusta. Last week, the results of a clinical study that was conducted in Augusta were published and the results – while brief – are very interesting.
The study involved a group of people with Parkinson’s who were treated with two different doses of Niacin (compared to a placebo treatment).
What is Niacin?
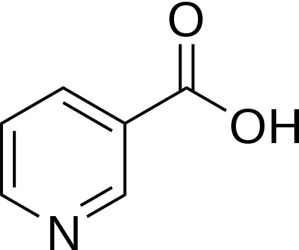
Niacin (also known as nicotinic acid) is an essential human nutrient – meaning that it is a nutrient required for normal physiological function which cannot be synthesized by the body, and is thus absorbed from one’s diet. Niacin is also a form of vitamin B3.
Niacin. Source: Wikipedia
It is used in medical practise, primarily to treat high blood cholesterol and pellagra (niacin deficiency).
Niacin is required for most cellular processes. Insufficient niacin in the diet can cause nausea, skin and mouth lesions, anemia, headaches, and tiredness. Long-term niacin deficiency results in the ‘four Ds’: dermatitis, diarrhea, dementia, and death.
How is Niacin associated with Parkinson’s?
Way back in 1979, a group of researchers noticed something interesting about the group of people with Parkinson’s that they were assessing:
Title: Niacin depletion in Parkinsonian patients treated with L-dopa, benserazide and carbidopa.
Authors: Bender DA, Earl CJ, Lees AJ.
Journal: Clin Sci (Lond). 1979 Jan;56(1):89-93.
PMID: 477187
In this study, the researchers noticed that the urinary levels of a chemical called N1-methylnicotinamide were as much as 40% reduced in the people with Parkinson’s who were being treated Levodopa – the standard treatment for condition. The reduction was not observed in people with Parkinson’s who were being treated with dopamine agonist drugs.
What on earth is N1-methylnicotinamide?
N1-methylnicotinamide is a product of a process that generates nicotinamide nucleotide, which is a key intermediate of nicotinamide adenine dinucleotide (NAD+) production.
And what is NAD+?
NAD+ is an important conveyor of hydrogen electrons for cells, and it is essential for the continued production of energy (ATP) by the mitochondria in cells. Without NAD+, things inside the cell start to go wrong very quickly. NAD+ is present in every cell and it is essential for normal functioning.
This video will help you to understand exactly what NAD+ does:
Now, NAD+ can be generated in two ways: from a particular cellular process or from dietary Niacin.
So what did the researchers result mean?
The 40% reduction in levels of N1-methylnicotinamide meant that cells inside the Levodopa treated people with Parkinson’s were no longer producing enough NAD+ using the particular cellular process approach. This situation suggested that the cells were relying more heavily on dietary Niacin for their production of NAD+.
Such was the burden, the investigators found that many of the participants taking Levodopa treatment could be “classified as ‘borderline deficient’ or ‘at risk’ of niacin deficiency”. The researchers concluded that clinicians should be keeping an eye open for signs of pellagra (or niacin deficiency) in people with Levodopa-treated Parkinson’s (but curiously, very few cases of pellagra have ever been reported in PD).
This is not the only association between Niacin and Parkinson’s though. A few years ago, researchers reported that there was an increase in the levels of receptors that niacin binds to in people with Parkinson’s:
Title: Upregulation of GPR109A in Parkinson’s disease.
Authors: Wakade C, Chong R, Bradley E, Thomas B, Morgan J.
Journal: PLoS One. 2014 Oct 17;9(10):e109818.
PMID: 25329911 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers looked at what was happening to GPR109A inside people with Parkinson’s.
What is GPR109A?
GPR109A is a G protein-coupled anti-inflammatory receptor.
And what on earth does “G protein-coupled anti-inflammatory receptor” mean?
Getting a signal from outside of a cell into the interior is a complicated affair.
There are numerous ways to do it, but one of the most common involves ‘G-proteins‘. G-proteins act as molecular switches.
G-proteins are located inside the cell membrane and are activated by G-protein-coupled receptors (like GPR109A). When a signalling molecule binds to the G-protein-coupled receptor on the outside of the cell membrane, the portion of the receptor inside the cell activates the G-protein which then starts of a chain of events resulting in the signal being passed on.

The life cycle of a G protein. Source: Bio1151
In the case of GPR109A, activation of the G protein causes a decrease in cyclic adenosine monophosate (cAMP) production. cAMP is a “second messenger” that plays a key role in regulating of many biologic responses inside us, including inflammation. By reducing cAMP, one reduces the level of inflammation.
So the take home message is: GPR109A is a receptor that – when activated – helps to reduce inflammation.
GPR109A can be found in blood cells that respond to infections (macrophages and neutrophils), as well as organs and tissues. And the anti-inflammatory role of GPR109A is well-established (Click here to read more about this).
And niacin (also known as nicotinic acid) acts as an agonists of GPR109A, which helps to reduce inflammation (Click here to read more about this).
What is an agonists?
An agonist is a drug that binds to and activates a particular receptor, while an antagonist is a drug that binds to and blocks the activation of a particular receptor.
On the surface of a cell, there are lots of receptors which act as switches for certain biological processes to be initiated. Receptors will wait for a protein to come along and activate them or alternatively block them. The activators are called agonists, while the blockers are antagonists.

Agonist vs antagonist. Source: Psychonautwiki
Ok, so what did the researchers discover about GPR109A in people with Parkinson’s?
They found that GPR109A levels in the blood and the substantia nigra – the region of the brain that suffers the greatest loss of dopamine producing neurons – are higher in people with Parkinson’s. In the graph below, you can see the difference in GPR109A levels in the blood from young subjects (the lime green bar), age-matched controls (Pink), and people with Parkinson’s (blue):
And in the image below, sections of brain tissue have been stained with dyes that label GPR109A (in red), microglia cells (green), and the nucleus of neurons (blue). You can hopefully see that there is more red and green in the section from the Parkinson’s (PD) brain than the age-matched control:
Source: NCBI
Thus, this result suggests that people with Parkinson’s have higher demands for niacin in order to help reduce ongoing inflammation. And if we consider that people being treated with Levodopa have even more increased needs for niacin, you can start to see why some members of the research community are interested in studying niacin and Parkinson’s.
And there are suggestions that this need for niacin could be beneficial in genetic forms of Parkinson’s:
Title: Enhancing NAD+ salvage metabolism is neuroprotective in a PINK1 model of Parkinson’s disease<
Authors: Lehmann S, Loh SH, Martins LM.
Journal: Biol Open. 2016 Dec 23. pii: bio.022186.
PMID: 28011627 (this article is OPEN ACCESS if you would like to read it)
In this study, the researchers analysed flies with genetic mutations in the Parkinson’s associated PINK1 gene. They found that PINK1 mutant flies have decreased levels of NAD+ and problems with their mitochondria (the power stations of each cell – Click here to read a previous post on PINK1 and mitchondria).
The researchers were curious to determine if a diet supplemented with compound that can help increase the production of NAD+ would rescue the mitochondrial defects observed in the PINK1 mutant fly. Specifically, they fed the flies a diet high in the NAD+ precursor nicotinamide (being a precursor means that nicotinamide can be made into NAD+ once inside a cell). They found that not only did nicotinamide rescue the mitochondrial problems in the flies, but it also protected neurons from degeneration.
I thought this post was about Niacin. Why are we talking about nicotinamide?
Niacin – like nicotinamide – is also a precursor of NAD+. And in their discussion of the study, the researchers noted that a high level of dietary niacin has been associated with a reduced risk of developing Parkinson’s disease (Click here and here for more on this).
Interesting. Have their ever been any clinical studies of niacin in Parkinson’s?
Until last week, there have only really been case studies (as far as I’m aware – happy to be corrected on this).
Title: Low-dose niacin supplementation modulates GPR109A, niacin index and ameliorates Parkinson’sdisease symptoms without side effects.
Authors: Wakade C, Chong R, Bradley E, Morgan JC.
Journal: Clin Case Rep. 2015 Jul;3(7):635-7.
PMID: 26273459 (This report is OPEN ACCESS if you would like to read it)
In this case study, a 65-year-old man with Parkinson’s underwent 45 days of daily niacin supplementation. He was assessed at the end of the study, and then again at 90 days since starting the treatment regime.
Overall, the niacin treatment was well tolerated and no side effects were reported. In addition, the niacin treatment helped to reduce levels of GPR109A receptor (which we discussed above) – see graph below:
Source: NCBI
In addition to this, the clinical features of the man’s Parkinson’s were reported to have improved (according to UPDRS scores), as well as improvements in sleep quality and handwriting. Interestingly, these benefits disappeared when the niacin treatment was stopped for 3 months and the man was re-evaluated. This result suggests that niacin is having a symptomatic effect rather than doing anything robustly neuroprotective.
But this case study does not exist in isolation.
A gentleman with Parkinson’s was instructed to take niacin in order to treat high triglycerides (a type of fat found in blood), but the treatment had the additional effect of decreasing rigidity and bradykinesia. Ultimately the individual could not continue the treatment as they did not tolerate niacin so well (it had resulted in severe nightmares and skin rash – Click here to read more about this case study).
These case studies, however, suggested to researchers that niacin was of interest and worthy of further investigation in Parkinson’s.
Which brings us to the clinical study results that were published last week:
Title: Niacin modulates macrophage polarization in Parkinson’s disease.
Authors: Wakade C, Giri B, Malik A, Khodadadi H, Morgan JC, Chong RK, Baban B.
Journal: J Neuroimmunol. 2018 Jul 15;320:76-79.
PMID: 29759143 (This report is OPEN ACCESS if you would like to read it)
In this study, 46 people with Parkinson’s were given placebo, 100 mg niacin or 250 mg niacin for three months. The investigators were blind to who received which treatment. The average age of the participants was 62 years for the placebo group, 64 and 61 years for the 100mg and 250mg groups, respectively. And the average duration of disease was approximately 6 years for all the groups.
After three months, the investigators found improvements in the quality-of-life measures in the group treated with 100 mg niacin (compared to the placebo group), but more importantly they observed a shift in state of macrophage cells – from M1 to M2 state.
What are macrophage and what does M1 or M2 mean?
As we discussed in the previous post, Macrophage cells are blood cells that play an important role in the body’s response to injury or infection.
A macrophage seeking and reaching for pathogens. Source: Wikipedia
They are derived from monocytes, which are the ‘first responders’ of the immune system. Monocytes change into macrophage as they leave the blood system and enter damaged or affected area of the body.
They are very specialised cells involved in the detection, ‘phagocytosis‘ (the process of engulfing or consuming objects) and destruction of bacteria and other agents that are harmful to organism. Macrophages are utterly ruthless. They consume anything that disagrees with them. And once consumed, the pathogen is broken down and spat out.
A macrophage. Source: Meducator
And ‘M1 and M2 state’ mean?
Macrophages can be present in an M1 state (which is believed to be inflammatory) or an alternative M2 state (which is anti-inflammatory).
And the researchers found that 3 months treatment of 250mg niacin shifted the macrophages from a primarily inflammatory M1 state to an anti-inflammatory M2 state, as indicated in the graph below:
Source: Sciencedirect
This is an interesting result, which demands further investigation, and a larger double blind, randomised clinical study is now underway in Augusta. Approximately 80 participants will be randomly and blindly treated with either 250mg niacin or a placebo drug for 6 months (Click here to read more about this trial).
It will be interesting to see the results of that study which should be available in late 2019.
So does all of this research mean that I should rush out and gorge myself on this niacin stuff?
Before I answer this question: You should always discuss with your doctor before radically changing any aspect of your diet or treatment regime, as such changes can impact the efficiency of your current medication or worse cause unintended side effects. Please consult your physician before attempting anything.
That said: Increasing niacin levels in not something that should be rushed into. Rapidly increasing levels of niacin can result in ‘flushing’ – a blushing of the skin or sensation of heat – which indicates a temporary saturation of niacin. And this may not sound like a big deal, but the consequences for someone taking cholesterol-lowering drugs or blood thinners could be serious. Thus, it is generally recommended that niacin is increased slowly over a long period (weeks and months).
Other common side effects of high niacin intake include:
- mild dizziness
- heart racing
- itching, dry skin
- jaundice (yellowing of your skin or eyes)
- nausea, diarrhea, belching, gas
- muscle pain, leg cramps
- sleep problems (insomnia)
The recommended dietary allowance (RDA) for niacin is 16 mg/day for men and 14 mg/day for women. But increasing one’s daily intake of niacin is relatively easy, because there are many foods that have naturally high levels of niacin, such as:
- 1) Turkey. 1 breast: 101 mg (over 100% of your daily value (DV))
- 2) Chicken breast. 3 oz: 8.9 mg (44% DV)
- 3) Peanuts. 1 cup: 21.9 mg (over 100% DV)
- 4) Mushrooms. 1 cup: 7.6 mg (34% DV)
- 5) Liver. 1 slice: 11.9 mg (60% DV)
- 6) Tuna. 3 oz: 11.3 mg (56% DV)
- 7) Green peas. 1 cup: 3 mg (15% DV)
- 8) Grass-fed Beef. 3oz: 7.6 mg (36% DV)
- 9) Sunflower seeds: 1 cup: 3.8 mg (19% DV)
- 10) Avocado. 1 whole fruit: 3.5 (17% DV)
(Source of list)
So what does it all mean?
A recent clinical study evaluating the safety and tolerance of the essential nutrient niacin in people with Parkinson’s has reported an interesting anti-inflammatory result. Treatment with niacin for 3 month resulted in a shift in the macrophage (blood cells) from an inflammatory state to an anti-inflammatory state. A larger clinical study is now being set up which will hopefully replicate and expand on this finding.
If the new study validates the ‘anti-inflammatory’ result of the current pilot study, it could be interesting to have a close look at the treatment group. While the follow up study is still small (compared to a large phase III trial), it could be interesting to determine if certain individuals responded better to the niacin treatment than others. A careful assessment of those individuals may highlight features or biomarkers that could be used to better target niacin as a treatment for particular individuals.
Until the new clinical study results become available late next year (possibly 2020), it would be interesting to see more preclinical research on niacin in models of Parkinson’s. As you may have guessed from this post, at present very little preclinical research has actually been conducted on niacin in the context of PD…. which obviously makes it very difficult for a simple blog writer to provide any kind of opinion on niacin when readers ask 🙂
ADDENDUM: 28th May 2018
Special thanks to reader Dr Kevin McFarthing who pointed out another niacin-related report:
Title: Parp mutations protect against mitochondrial dysfunction and neurodegeneration in a PARKIN model of Parkinson’s disease
Authors: Lehmann S, Costa AC, Celardo I, Loh SH, Martins LM.
Journal: Cell Death Dis. 2016 Mar 31;7:e2166.
PMID: 27031963 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers who conducted the PINK1 research discussed in the post above also investigated another Parkinson’s-associated protein called PARKIN in the context of niacin. The investigators found that the loss of PARKIN affects NAD+ metabolism in flies. The levels of NAD+ are significantly decreased in flies with , but by increasing NAD+availability – via dietary supplementation of the NAD+ precursor nicotinamide (or NAM – a form of niacin) – they could increase NAD+ levels which rescued mitochondrial function and demonstrated neuroprotection of dopamine neurons.
Source: PMC
All of this research was conducted in flies, but the findings provide further preclinical evidence supporting niacin.
EDITOR’S NOTE: The information provided by the SoPD website is for educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. Please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Deadseamoringa

















Many thanks for this. There is consistent interest in the B vitamins among PWPs, suggesting that they do help. It would be great to have more studies and reviews.
LikeLike
Hi Kevin,
Glad you liked the post. Since posting this one there has been some demand for a thiamine/vitamin B1 post. It is definitely on the list of things to do.
Kind regards,
Simon
LikeLike
Hi Simon,
And thanks for the information on B3. Several researchers have been working with B3 but there are also several different forms of B3 and the effects received from them do vary. The infamous ‘Niacin flush’ is like a fresh case of prickly heat but there are people out there who enjoy the sensation and take big doses to ‘spring clean’ their arteries – no, I doubt that is a very sensible course of action to pursue.
Along with niacin and niacinamide is nicotinic acid and the relatively novel nicotinamide riboside. This latter version of B3 has had wonder drug status given to it by some (especially by companies that sell it!) yet it does not seem to work in young people. In my opinion the young, normal human body works perfectly well with basic B3 but the n riboside version of B3 may enhance some aspects of cellular metabolism (read: mitochondrial functions). Unfortunately it is expensive and the patent rights are still in force for the benefit of the manufacturer/retailer.
I would welcome fruit fly testing where this novel form of B3 could be used on ‘damaged’ flies to see if a quantifiable difference could be detected.
Okay, don’t want to say too much until more study of tests and trials have been located and studied – as Holmes said to Watson ‘I never guess!’ and that is what I would be doing by pre-empting results.
Keep well!
LikeLike
Hi Lionel,
Keeping well. Yourself? Given recently published research, nicotinamide riboside is going to get a post all of its own. It is near the top of my to do list (along with a hundred other things…).
Thanks for the comment.
Kind regards,
Simon
LikeLike
Hi Simon, thank you for another great article! I have a couple of basic question:
1). Why take Niacin vs Vitamin B3?
2). Would there be a benefit to taking vitamin B12 (based on clinical trials or case studies) as well?
3). Is there benefit to take Niagen vs Niacin?
Apologies in advance if this is covered and I missed it.
Thanks!
LikeLike
Hi Pamela,
Glad you liked the post – thanks for the interesting questions:
1. This is an easy one, because there is no difference between the two. Vitamin B3 comes in three forms: nicotinamide (niacinamide), niacin (nicotinic acid), and nicotinamide riboside. And all three forms are converted by your body into nicotinamide adenine dinucleotide (NAD). And as we discussed in the post above, NAD is present in every cell and it is essential for normal functioning.
2. B12 is interesting. Basically, most of the evidence published to date suggests that B12 is lower in folks with PD (both those treated with L-DOPA and those without). But interestingly, the research suggests that there is no difference in dietary intake of B12 between PwPs and controls, and no association between dietary B12 intake and risk of developing Parkinson’s (here is an example of that research: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4586528/). I am not aware of any completed clinical trials for B12 in PD. There were two planned studies (one at The Feinstein Institute For Medical Research in New York and another at Emory University in Georgia), but both of these were cancelled (for reasons unknown to me). The reason for considering B12 supplement is that L-DOPA treatment can lower one’s levels of B12 (https://www.ncbi.nlm.nih.gov/pubmed/17565222), but you should always discuss any changes in treatment regime with your doctor before proceeding.
3. As with question 1, this is an easy one to answer: Niagen (or synthetic nicotinamide riboside) and Niacin are both forms of vitamin B3. They both basically do the same thing (get converted to NAD). There are some subtle differences, but I am working on a post looking at new research on nicotinamide riboside in models of PD and I can address this matter in that post. The main issue for most people with using Niagen is the cost.
I hope all of this helps – please let me know if you have any further questions on it.
Kind regards,
Simon
LikeLike
Here
https://www.cell.com/cell-reports/fulltext/S2211-1247(18)30742-3
is another study on B3 and PD.
(The DOI
https://doi.org/10.1016/j.celrep.2018.05.009
given there does not work (yet).)
LikeLike
Hi ZZ,
Many thanks for that!
Simon
LikeLike
Simon,
Have you seen this study? Also curious why no mention of NR versus NAM. I realize that NR is “hyped” but you can certainly stick to the facts when discussing it.
The NAD+ Precursor Nicotinamide Riboside Rescues Mitochondrial Defects and Neuronal Loss in iPSC and Fly Models of Parkinson’s Disease
https://www.cell.com/cell-reports/fulltext/S2211-1247(18)30742-3
Joe
LikeLike
Hi Joesph,
Thanks for your comment and for the link. A post on B1 (much demanded by readers) and a post on the new NR research have been added to my list of things to do. The NR work in particular is really interesting.
Kind regards,
Simon
LikeLike
Sigh. I began taking B3 (IR) 2 grams per day after my GP found 25% and 33% plaque build up in my carotids at the age of 53 (64, now, BTW). My HDL is close to 70, about the same as my LDL (thanks to LD Lipitor). TG are almost unmeasurable. I got used to the flushing by titrating my dosages.
There have been studies showing both pros and cons for this approach. For me, however, my plaque has shrunk to around 20% on one side, and stabilized at 25% on the other. Neither number is a cause for concern according to my cardiologist, especially since my yearly Stress tests are uniformly great for a man my age.
If Niacin is Neuroprotective, then Praise the Lord! Otherwise, my own personal experience leads me to believe that it is at least Cardioprotective, and maybe protects against ischemic strokes.
As always, keep up the great work!
LikeLike
Hi Simon, Thanks for your site! Here are some items of potential interest:
Poly(ADP-ribose) drives pathologic α-synuclein neurodegeneration in Parkinson’s disease
Tae-in Kam et al.
http://science.sciencemag.org/content/362/6414/eaat8407
https://medicalxpress.com/news/2018-11-road-cell-death-parkinson-disease.html
Abstract paraphrased: “Pathologic α-synuclein (that which is bound more strongly together with PAR) activates PARP-1 which produces more PAR and drives cell death via parthanatos. In a feed-forward loop, PAR converted pathologic α-synuclein to a more toxic strain. PARP inhibitors or genetic deletion of PARP-1 prevented pathologic α-synuclein toxicity.”
Three anti-cancer PARP inhibitor drugs stopped this process and so may be useful in combatting PD: Veliparib (experimental), Rucaparib and Atalazoparib (both available from single suppliers).
Googling nicotinamide PARP indicates that nicotinamide (vitamin B3) is also a PARP inhibitor, which is how I found this page.
https://medicalxpress.com/news/2018-10-appendix-potential-parkinson-disease.html
The vermiform appendix impacts the risk of developing Parkinson’s disease
Bryan A. Killinger et al.
http://stm.sciencemag.org/content/10/465/eaar5280 (Open access.)
“. . . the human appendix contains an abundance of misfolded α-synuclein and that removal of the appendix decreased the risk of developing PD. The appendix of both PD cases and healthy individuals contained abnormally cleaved and aggregated forms of α-synuclein, analogous to those found in postmortem brain tissue from patients with PD. Furthermore, α-synuclein derived from the appendix seeded rapid aggregation of recombinant α-synuclein in vitro. In two large-scale epidemiological studies, the authors demonstrated that an appendectomy occurring decades prior reduced the risk of developing PD, suggesting that the appendix may be implicated in PD initiation.”
People who have had their vagus nerve removed are also at lower risk for PD. So it could be argued that a significant pathway to full PD pathology starts in the gut.
“This signifies that α-synuclein aggregation in the appendix is a common phenomenon (much more common than PD) and therefore is not likely to be implicitly disease causing. Rather, it suggests that other biological processes that suppress the spread of α-synuclein aggregates or are responsible for the clearance of α-synuclein aggregates in the appendix may be crucial determinants in PD development. Hence, the abundance of α-synuclein aggregates in the healthy appendix signifies that, in certain individuals, there might be a “secondary hit” that aids in the accumulation and uninhibited spread of α-synuclein from the gut to the brain, eventually causing PD. In addition, removal of the appendix may reduce GI tract inflammation and thereby reduce systemic inflammatory cytokines, which contribute to PD as recent studies show.
Also some research indicating dietary factors which increase the risk of PD:
https://www.ncbi.nlm.nih.gov/pubmed/8797457 1996
Low beta-carotene and ascorbic acid. Low niacin is especially significant.
https://www.ncbi.nlm.nih.gov/pubmed/9918341 1999
Low intake of a variety of niacin-rich foods. Exposure to pesticides. Male carpenters and female cleaners at higher risk.
https://www.ncbi.nlm.nih.gov/pubmed/20188745 2010
“These findings suggest that nicotinamide has a protective effect against PARP-1-induced astrocyte death and that its transporter-mediated uptake, which is extracellular pH-sensitive and common to N-methylnicotinamide, is critical for prevention of PARP-1-triggered cell death.”
https://www.ncbi.nlm.nih.gov/pubmed/12796527 2003
High iron and/or manganese, especially both.
https://www.ncbi.nlm.nih.gov/pubmed/29681846 2018
High iron (mouse study).
https://www.ncbi.nlm.nih.gov/pubmed/16499406 2005
Manganese from steel welding fumes.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3230726/ 2011
Manganese may work with α-synuclein to kill neurons.
https://www.ncbi.nlm.nih.gov/pubmed/29229294 2018
Mouse model: High intake of omega 3 fatty acids enabled the mice to survive 51% longer.
LikeLike
Very interesting stuff, Robin. Makes me wonder if the so-called unnecessary appendix is likewise a garbage collector for misfolded proteins, that maybe st some point gets overloaded and starts dumping bad stuff into the system. If one has tisk factors, maybe removing the appendix could be a preventative action. Or maybe not?
In any case, I’ll keep taking my medicinal doses of B3 to keep my HDL nice and high!
LikeLike
One more item of potential interest:
https://medicalxpress.com/news/2018-10-cooling-brains-parkinson.html
Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice
R. Gordon el al. 2018-10-31
http://stm.sciencemag.org/content/10/465/eaah4066
A small molecule MCC950 (https://www.nature.com/articles/s41598-018-26775-w) inhibits inflammatory responses in cells including microglia. “Oral treatment with an inflammasome inhibitor improved motor performance and reduced neuroinflammation, neurodegeneration, and α-synuclein accumulation in mouse models of α-synuclein–mediated toxicity. The results suggest that systemic delivery of inflammasome inhibitors might have therapeutic effects in PD.”
LikeLike
Hi Simon,
The mentioned trial in the article apparently finished in April of this year. Are you aware if the study results are going to be published?
LikeLike
High comorbidity of seborrhoeic dermatitis is reported in PD but I wonder if, in some cases, this is actually a manifestation of subclinical Pellagra?
Thoughts?
LikeLike