|
New research provides some interesting insight into particular cellular functions – and possibly sleep issues – associated with Parkinson’s. Researchers in Belgium have recently published interesting findings that a genetic model of Parkinson’s exhibits sleep issues, which are not caused by neurodegeneration, but rather neuronal dysfunction. And as a result, they were able to treat it… in flies at least. In today’s post, we will review this new research and consider its implications. |
Source: Dlanham
I am a night owl.
One that is extremely reluctant to give up each day to sleep. There is always something else that can be done before going to bed. And I can often be found pottering around at 1 or 2am on a week night.
As a result of this foolish attitude, I am probably one of the many who live in a state of sleep deprivation.
I am a little bit nervous about doing the spoon test:
But I do understand that sleep is very important for our general level of health and well being. And as a researcher on the topic, I know that sleep complications can be a problem for people with Parkinson’s.
What sleep issues are there for people with Parkinson’s?
We discussed this topic in a recent post (click here to read that post), but one large Italian study found that sleep related issues could be broken down into:
- REM sleep behaviour disorder (29.6% of cases)
- Insomnia (36.8%)
- Excessive day time sleepiness (21.2%)
- Restless legs (15.2%)
These results have been replicated numerous times. In fact, the incidence of sleep problems in Parkinson’s is generally stated as occurring in 40% to 90% of cases (Click here for a good review on the topic).
What is REM sleep behaviour disorder?
Rapid eye movement (REM) sleep behaviour disorder (sometimes simply referred to as RBD) is a condition that can often proceed Parkinson’s, in which the sufferer will act out their dreams. Below is a video of Prof Jose Loredo – a sleep expert – explaining what RBD is exactly:
And here is an example of what REM sleep behaviour disorder looks like. The individual being observed is asleep throughout the entire video, but half way through he will begin reacting to a scary dream he is having:
The exact cause of RBD is unknown, but the condition is association with various degenerative neurological conditions, such as Parkinson’s, Multiple System Atrophy, and Lewy body dementia. This association has given rise to the theory that RBD may result from the death of neurons in the brain stem that help to control the body during sleep. These cells normally prevent the body from physically responding to the events in dreams, but in their absence there is nothing stopping the body from reacting.
Normal REM sleep VS RBD. Source: Smrv
Are all sleep issues in Parkinson’s associated with cell loss?
This is a really interesting question.
And recently, there has been some research published that suggests that sleep problems can arise from neuronal dysfunction (as opposed to neurodegeneration).
Here is the research report:
Title: ER Lipid Defects in Neuropeptidergic Neurons Impair Sleep Patterns in Parkinson’s Disease
Authors: Valadas JS, Esposito G, Vandekerkhove D, Miskiewicz K, Deaulmerie L, Raitano S, Seibler P, Klein C, Verstreken P.
Journal: Neuron. 2018 Jun 1. pii: S0896-6273(18)30420-3.
PMID: 29887339
In this study, the researchers from the Katholieke Universiteit Leuven and VIB – the Flanders Institute for Biotechnology wanted to investigate sleep disturbance in models of Parkinson’s.
And for their model of Parkinson’s, the investigators chose to use a flies.

Flies. Source: TheConservation
Which kind of begs the obvious question:
Do flies actually sleep???
Yes they do.
Flies are one of the smallest members of the animal kingdom that requires sleep, and they also have different stages of sleep, in a very similar fashion to humans.
This video discusses sleep in flies further:
But flies don’t close their eye! And they are so small. Do they snore? How on earth do you measure sleep in flies?
A fly is considered to be asleep if it has been inactive for 5 min or more. And besides a higher threshold to arousal, sleeping flies do not move and inactivity over a 5-min period is regarded as a good measure of sleep.
Scientists measure these periods of in activity by placing individual flies into closed glass tube that have multiple infrared beams passing through them. Each time a beam is crossed, the fly is considered to be active. For example:
Measuring sleep in flies. Source: Animalphys4e
And these tubes are kept in an incubator on a strict light/dark schedule. Periods of inactivity (sleep) can be measured across a period of 24 hours.
Researchers use these periods of inactivity as a representation of sleep and a lot of research has now been conducted on the nature of sleep in flies (Click here for an example).
Ok, so what did the new research find?
Before we get to the research, there is one more thing to explain about this study, and that is: the model of Parkinson’s.
For their study, the researchers chose to investigate flies with genetic mutation in their PINK1 or PARKIN genes.
What are PINK1 and Parkin?
Approximately 10-20% of Parkinson’s cases are associated with particular genetic variations (or mutations) that render people vulnerable to developing the condition. These mutations occur in regions of DNA (called genes) that provide the instructions for proteins.
PINK1 and PARKIN are two genes that can have Parkinson’s associated genetic mutations.
What do the PINK1 and PARKIN genes do?
The proteins produced by the PINK1 or PARKIN genes appear to have many different functions, but their roles in the process of mitophagy are relatively well understood, and believed to be involved with their association to Parkinson’s.
What is mitophagy?
Mitophagy involves the disposal of old or dysfunctional mitochondria.
Mitochondria – you may recall from previous SoPD posts – are the power stations of each cell. They help to keep the lights on. Without them, the party is over and the cell dies.
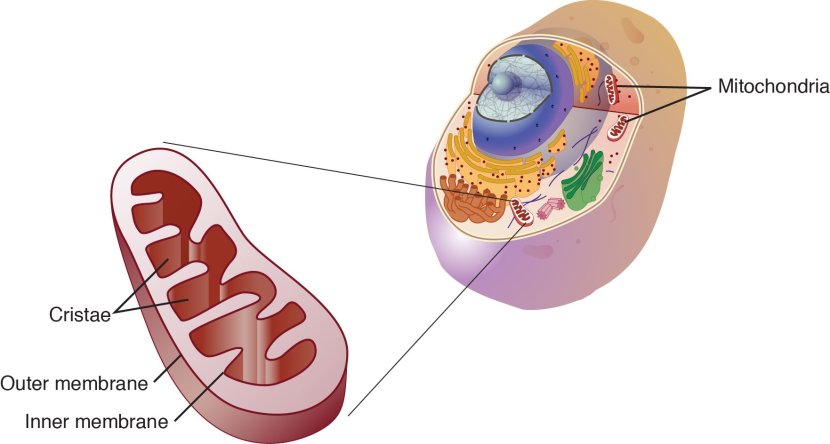
Mitochondria and their location in the cell. Source: NCBI
You may remember from high school biology class that mitochondria are tiny bean-shaped objects within the cell. They convert nutrients from food into Adenosine Triphosphate (or ATP). ATP is the fuel which cells run on. Given their critical role in energy supply, mitochondria are plentiful (some cells have thousands) and highly organised within the cell, being moved around to wherever they are needed.
Like you, me and all other things in life, mitochondria have a use-by date.
As mitochondria get old and worn out (or damaged) with time, the cell will dispose of them via mitophagy.
What do PINK1 and PARKIN do in mitophagy?
PINK1 acts like a kind of handle on the surface of mitochondria. In normal, healthy cells, the PINK1 protein attaches to the surface of mitochondria and it is slowly absorbed until it completely disappears from the surface and is degraded. In unhealthy cells, however, this process is inhibited and PINK1 starts to accumulate on the outer surface of the mitochondria. Lots of handles poking out of the surface of the mitochondria.
Now, if PINK1 is a handle, then PARKIN is a flag that likes to hold onto the PINK1 handle. While exposed on the surface of mitochondria PINK1 starts grabbing the PARKIN protein. This pairing is a signal to the cell that this particular mitochondrion (singular) is not healthy and needs to be removed. The pairing start the process that leads to the development of the phagophore and eventually mitophagy.

Pink1 and Parkin in normal (right) and unhealthy (left) situations. Source: Hindawi
In the absence of normal PINK1 or PARKIN proteins, there is no handle-flag system and old/damaged mitochondria start to pile up. They are not disposed of appropriately and as a result the cell gets sick and ultimately dies.
Mitophagy. Source: Frontiersin
People with particular mutations in the PINK1 or PARKIN genes are vulnerable to developing an early onset form of Parkinson’s. It is believed that the dysfunctional disposal of (and accumulation of) old mitochondria are part of the reason why these individuals develop the condition at such an early age.
So what happens to sleep in flies with no PINK1 or PARKIN?
The researchers found that two features of fly sleep patterns were particularly disrupted in flies with PARKIN or PINK1 genetic mutations. Those two being:
- the anticipation of dawn
- the fragmentation of sleep
And this is demonstrated in the image below:
Source: Neuron
In the image, you can a 24 hour representation of activity of flies. Each coloured vertical bar represents a 5 minute period of activity as measured by number of infrared beams being broken; and each horizontal row of coloured bars and blank spaces represents one fly in the study. The flies are divided into three groups: normal/control flies (blue), those with PARKIN and PINK1 mutations (red bars and pink bars, respectively), and those with PARKIN and PINK1 mutations that have ‘been rescued’ (a functional PARKIN or PINK1 gene has been inserted – green bars and purple bars, respectively).
What you can see is that during the ‘DARK’ phase, the normal/control flies (in blue) have very busy periods of activity around the ‘sunrise and sunset’ periods of the LIGHT/DARK periods of their day. This is less apparent in the PARKIN and PINK1 mutant flies (red bars and pink bars, respectively).
This is the issues with ‘the anticipation of dawn‘ aspect of sleep mentioned above.
In addition, during the DARK period, there are lots of blank spaces which represent periods of inactivity (or sleep) in the control flies. Now, compare that inactivity of the controls during the DARK period, with the more active PARKIN and PINK1 mutant flies (red bars and pink bars, respectively – just below the blue control flies). You will hopefully see that during the DARK period there are more red and pink bars compared to blue bars.
This is the issues with ‘the fragmentation of sleep‘ aspect of sleep mentioned above.
Curiously, the total amount of time flies with PARKIN or PINK1 genetic mutations spent asleep was no different to control flies – their sleep was simply more fragmented.
By inserting functional versions of the PARKIN and PINK1 genes along side the mutant versions of the genes in these flies, the researchers found that they could rescue these sleep issues. Note that the patterns of the green and purple bar resemble the blue bar patterns more than the red and pink bars, suggesting better quality of sleep.
Interesting. So what did the researchers do next?
They further analysed the defect in flies by limiting the mutation to specific populations of neurons in the brain, and by using this approach (which must have been A LOT of work) they narrowed the problem down specific clusters of neurons in the brain.
And when the researchers looked closer at those populations of neurons, they found that those cells were have trouble releasing neuropeptides – small compounds which act like messengers (or neurotransmitters) between cells.
Not only were the cells releasing less neuropeptides, they were also producing less.
The production and release of neuropeptides. Source: Isyslab
There were less neuropeptides floating around inside the And this observation led the investigators to focus their attention on the endoplasmic reticulum.
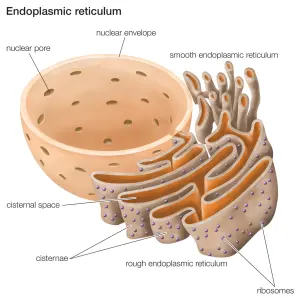
What is the endoplasmic reticulum?
The endoplasmic reticulum (or ER) is the assembly line where proteins are produced in a cell. It is closely attached to the nucleus of the cell.
The endoplasmic reticulum. Source: Britannica
The nucleus is where the blue prints/designs for each protein are kept in the form of DNA. A template of each plan for a protein can be generated (in the form of RNA), and that is converted (via a process called translation) into protein. A large part of that protein production process is conducted in the endoplasmic reticulum.
This video explains exactly what the ER does:
When the researchers looked at the ER of flies with PARKIN or PINK1 genetic mutations, they found an increased amount of unprocessed and partially processed neuropeptides. This finding suggested to them that there was a protein production issue in these mutant flies.
But this raised the question: How?
How could a pair of proteins involved in removing mitochondria be preventing proteins from being properly produced???
So what did the researchers do to answer this question?
Why, they looked at the mitochondria of course.
And while they did not observe any major morphological differences in the shape of the mitochondria of flies with PARKIN or PINK1 genetic mutations, they did notice more mitochondria making contact with the ER.
Note in the image above the close proximity of the ER (green) and the mitochondria (red) in the PARKIN cell (right), compared to the more diffuse distribution of the control cell (left).
To determine if this increased contact between mitochondria and the ER was relevant to human (or just specific to flies), the investigators grew iPS cells from people with PARKIN or PINK1-associated Parkinson’s.
What are iPS cells?
We live in an age where skin cells (or fibroblasts) can be converted into neurons via a trick of molecular biology. Those skin cells became induced pluripotent stem (or iPS) cells (which you can read about by clicking here). And these iPS cells can be subsequently encouraged to become any cell that you want – such as a neuron (or brain cell) – which gives rise to the possibility of more personalised approaches to medicine.
Making iPS cells. Source: learn.genetics
When the investigators grew neurons from iPS cells made from people with PARKIN or PINK1 genetic mutations, they also observed increased contact between mitochondria and the ER in those cells (compared to control iPS cells).
But what does increased contact between mitochondria and the ER mean?
This was the next question the researchers asked and they found that increased contracts between the mitochondria and the ER in PARKIN mutants resulted in excess amounts of a protein called Phosphatidylserine being transfered to mitochondria from the ER.
And it was this abnormal trafficking of Phosphatidylserine in these neurons that was disrupting the production of the neuropeptides.
What is Phosphatidylserine?
Phosphatidylserine is a phospholipid, and phospholipids are a class of lipids that are a major component of all cell membranes. Phosphatidylserine has a number of different functions in the body, from helping the brain metabolise glucose to aiding in the release of chemical messengers (neurotransmitters)
Phosphatidylserine. Source: Bestnootropicsnow
Now the interesting part of this whole story is that when the investigators started to experiment with Phosphatidylserine, they observed something amazing:
The depletion of Phosphatidylserine is certain populations of cells in the brain of normal flies would result in a very similar pattern of sleep to that observed in the PARKIN and PINK1 mutant flies.
And then the researchers performed the opposite experiment: they fed PARKIN and PINK1 mutant flies with food supplemented with Phosphatidylserine, and they found that this treatment could rescue the sleep patterns of the flies. In fact, after just three days of treatment, significant improvements were observed:
The researchers concluded their study by writing that ‘the sleep patterns and circadian disturbances in Parkinson’s disease models are explained by excessive ER-mitochondrial contacts, and blocking their formation or increasing phosphatidylserine levels rescues the defects‘, but they are quick to note that more research is required before any human-related conclusions could be made.
And follow-up the research may be hampered by troublesome mice.
In their study, the researchers examined sleep in mice with PINK1 mutants (using 24-hr activity monitoring), but they failed to detect any consistent defects when compared to control mice. And this is one of the issues with Parkinson’s research – none of the genetic mouse models we currently have display a combination of sleep fragmentation, excessive daytime sleepiness, or REM sleep behaviour disorder (Click here to read more about sleep in this regard).
As one of the researchers in the study, Dr Patrik Verstreken, has suggested in an interview about the research, “Translating the phosphatidylserine experiments is not straightforward, as similar sleep manifestations are absent in mouse models of Parkinson’s disease. The good news is that phosphatidylserine is already marketed as a food supplement, so if we can prove efficacy in humans, this would be very good news. There are still a lot of questions though. For example, we don’t know if phosphatidylserine could be delivered to the brain in humans, or at which dose.” (Source).
EDITOR’S NOTE: Phosphatidylserine is a widely available supplement that is considered safe to take in reasonable doses. Soy-derived Phosphatidylserine is designated ‘Generally Recognized As Safe’ by the US FDA, but Phosphatidylserine has not been approved for any medicinal use by the FDA. If any readers are considering use of Phosphatidylserine, PLEASE consult with your doctor/clinician before making any changes to your treatment regime. The new research results presented on this post need to be independently replicated and then tested in controlled clinical trials before any conclusions with regards to their relevance in humans can be made. To date, the effect only works in flies.
So what does it all mean?
New research points towards cellular processes that may be affecting sleep behaviour in fly models of Parkinson’s. The research is really interesting for two main reasons:
First, the results point towards a novel mechanism by which two Parkinson’s-associated proteins could be indirectly influencing cellular processes that do not relate to their primary (perceived) functions, but could be impacting an important aspect of behaviour. And this is particularly important from the standing point of Parkinson’s.
Another important observation from this study is that issues in sleep patterns could be partly caused by neuronal dysfunction – and not solely neurodegeneration – which in turn implies that it could be corrected, perhaps by phosphatidylserine. This idea still requires a great deal of further research to determine if it is valid in humans.
While all of this research is very interesting, I doubt that it will alter my silly nocturnal activities.
I can always go to bed early tomorrow night (NOTE: I say this every night!).
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from pexels











Thanks, Simon. It would be very interesting to see if phosphatidyl serine rescues other symptoms in the Drosophila PINK and Parking models, rather than trying to transfer the sleep investigation to mice. There could well be other existing symptom models in higher animals that could be tested.
LikeLike
Hi Kevin,
Thanks for the comment – glad you liked the post. I didn’t really want to complicate things here by delving too much into all the different neuropeptides, but yes it would be really interesting to explore whether the increase in ER-mitochondrial contacts phenomenon occurs in other cell types in the PINK1- or PARKIN- mutant setting, and what effect this could have on different peptides and neurotransmitters (not only in flies, but also human cells). Like all good research, this study has opened many doors.
Whether phosphatidylserine can rescue any effects observed in those other cell types would be interesting to determine as well. There is actually a really interesting story to be told about phosphatidylserine (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4183298/), but this post was already ridiculously long enough. A topic for another post perhaps.
Kind regards,
Simon
LikeLike
Very beautifully written article and so full of tidbits of education that I thoroughly enjoyed. I was thinking of starting a similar blog but then I ran into your blog and I just share your posts now as I cannot do this good of a job with my posts.
LikeLike
Hi Danish,
Thanks for your comment and kind words about the website. Greatly appreciated! I really like your website as well (https://danishbhatti.com/), particularly the podcast component (I myself have a face for radio and a voice for silent film). The more researchers/clinicians who can provide their thoughts regarding all the ongoing PD research, the better off the PD community will be. So by all means, keep up the good work.
Kind regards,
Simon
LikeLike
Simon
Very interestig piece!! Do the researchers porpose any furhter work on other models or humans?
LikeLike
Hi Keith,
Thanks for your comment. Yes, the researchers are seeking to follow up this research. Both the basic biology (does the ER-mitochondria phenomenon occur in other cell types? If so, which messenger proteins are affected?) and the rescue aspect (can Phosphatidylserine help in those other cell types as well?). With regards to the translational aspect, I am not sure. Phosphatidylserine is a cheap, safe supplement and sleep is easily assessed so I don’t see why it could not be tested in human rather quickly. The only things I would want to see before that leap would be a) independent replication, and b) other models of PD (Pink1 and Parkin mutations are rare).
Kind regards,
Simon
LikeLike