|
This week a biotech company called Voyager Therapeutics provided an update regarding a gene therapy approach for people with severe Parkinson’s. Gene therapy is an experimental therapeutic approach that involves inserting new DNA into cells using a virus. The introduced DNA can help a cell to produce proteins that it usually wouldn’t produce, and this can help to alleviate the motor features of Parkinson’s. In today’s post we will discuss what gene therapy is, what Voyager Therapeutics is trying to do, and outline what their update reported. |
There are 4 phases to the clinical trial process of testing new treatment for use in humans:
- Phase I determines if a treatment is safe in humans (this is conducted in an ‘open label’ manner)
- Phase II ‘double blindly’ assesses in a small cohort of subjects if the treatment is effective
- Phase III involves randomly and blindly testing the treatment in a very large cohort of patients
- Phase IV (often called Post Marketing Surveillance Trials) are studies conducted after the treatment has been approved for clinical use
(‘Open label’ refers to both the investigator and the participants in a study knowing what treatment is being administered; while ‘double blind’ testing refers to studies in which the participants and the investigators do not know whether the participant is receiving the active treatment or an inert control treatment until the end of the study).
Based on the successful completion of their Phase I clinical trials for their gene therapy treatment called VY-AADC (Click here to read more about this), Boston-based biotech firm Voyager Therapeutics approached the US Food and Drug Administration (FDA) with the goal of shifting their clinical trial programme into Phase II testing.
What is gene therapy?
The gene therapy involves introducing a piece of DNA into a cell which will cause the cell to produce proteins that they usually do not (either by nature or by mutation). The DNA is artificially inserted into cells and the cell’s protein producing machinery does the rest.
Source: Yourgenome
How does gene therapy work?
The introduction of the section of DNA – which provides the instructions for making a particular protein – is usually achieved using carefully engineered viruses. The viruses have had all the disease causing components removed, allowing us to use the virus as an efficient biological delivery system. Viruses by their very nature are very good at infecting cells, so if we remove the disease causing components, what is left is a very effective system of getting whatever you want into a cell.
Taking this approach one step further, we can take sections of DNA that contain the genes (these are the instructions for making proteins) involved with the production of a chemical called dopamine and insert them into our empty virus.

Gene therapy for Parkinson’s. Source: Wiki.Epfl
Dopamine is a chemical in the brain whose levels are badly affected by Parkinson’s. A severe reduction in dopamine levels results in the use of L-dopa as a treatment for Parkinson’s, as it helps to resort dopamine levels. By injecting a virus with dopamine-production associated DNA into the brain, we can produce dopamine in any infected cells (it’s slightly more complicated than that, but you get the basic idea).
So what is the Voyager trial trying to do?
Voyager Therapeutics‘s gene therapy product, VY-AADC is an adeno associated virus (or AAV) that carries a piece of DNA which contains the instructions (or a gene) for making a protein called Aromatic L-amino acid decarboxylase (or AADC).

AAV Viruses. Source: HuffingtonPost
And yeah, I know what you are going to ask next:
What is AADC?
AADC is the enzyme involved in the production of the chemical dopamine. Specifically, AADC converts the chemical L-dopa into dopamine. L-dopa is naturally produced in the brain from a protein called Tyrosine which is absorbed into brain cells from the blood. L-dopa is also the basis of many treatments for Parkinson’s (such as Levodopa – commercial versions of Levodopa include ‘Sinemet’).
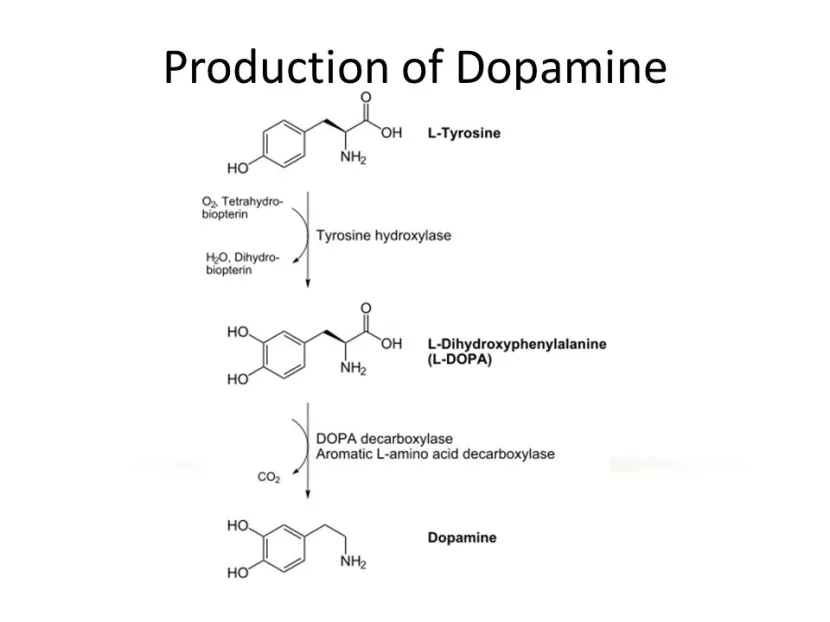
The production of dopamine. Source: Slideplayer
So AADC helps to produce dopamine, but why is dopamine important?
Dopamine is a chemical in the brain that helps us to move freely. Without it, our movements become inhibited – as in the case of Parkinson’s.
The majority of the dopamine produced in your brain is made in a region called the substantia nigra, deep inside you brain. These dopamine producing cells also generate another chemical called neuromelanin, which has a dark colouration to it – making it visible to the naked eye. As you can see in the image below, the substantia nigra region is easy to see on the section of healthy control brain on the left, but less visible on the section of brain from a person who passed away with Parkinson’s.
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinson’s disease brain (right). Source:Memorangapp
The dopamine neurons of the substantia nigra release their dopamine in different areas of the brain. The primary regions of that release are areas of the brain called the putamen and the Caudate nucleus. The dopamine neurons of the substantia nigra have long branches/projections (called axons) that extend a across the brain to the putamen and caudate nucleus, so that dopamine can be released there.
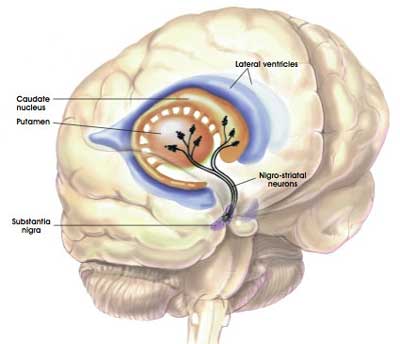
The projections of the substantia nigra dopamine neurons. Source: MyBrainNotes
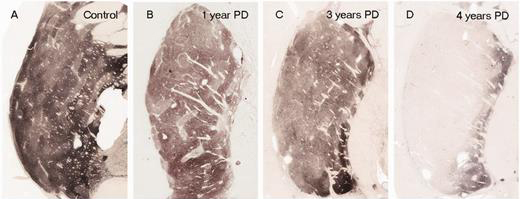
In Parkinson’s, these ‘axon’ extensions that project to the putamen and caudate nucleus gradually disappear as the dopamine neurons of the substantia nigra are lost. When one looks at brain sections of the putamen after the axons have been labelled with a dark staining technique, this reduction in axons is very apparent over time, especially when compared to a healthy control brain.
The putamen in Parkinson’s (across time). Source: Brain
So how does the VY-AADC virus help?
By injecting VY-AADC into the putamen of people with advanced Parkinson’s, Voyager is trying to make cells in that region (the putamen) start to produce AADC (which they usually don’t) and this will allow (in the use of Levodopa treatment) for the production of dopamine in the location where it is normally released by the (now absent) dopamine neurons. And this will hopefully help alleviate the motor features of the condition.
It must be understood, however, that the approach that Voyager Therapeutics is trialling here will not cure Parkinson’s, but it may make life a lot easier for those affected by it.
So what does the update from Voyager Therapeutics say?
The company made two announcements in their press release (Click here to read it).
Firstly, they announced that they had received feedback from a Type C meeting that they had had with the U.S. Food and Drug Administration (FDA).
What is a Type C meeting?
The FDA collects fees from drug manufacturers to fund the ‘New Drug Approval’ (or NDA) process, according to the Prescription Drug User Fee Act (PDUFA) of 1992. In exchange for collecting those fee, however, the FDA is expected to meet certain requirements related to the speed of certain activities within the NDA review process. One of those requirements is the holding of meetings with groups taking a drug through the clinical trial process.
The FDA. Source: Vaporb2b
There are three types of meetings (A, B, & C), and the meeting type is subject to different procedures.
- Type A meetings are for an otherwise stalled drug development program to proceed.
- Type B meetings are focused on pre-investigational new drug applications, certain end-of-phase 1 or end-of-phase 2 and pre-phase 3 issues (including risk evaluation and mitigation strategies).
- Type C meetings deal with issues regarding the development and review of a product, that do not fall under Type A & B.
Voyager had previously submitted questions to the FDA in preparation for a planned Type C meeting. The meeting is to discuss the regulatory pathway for VY-AADC for the treatment of Parkinson’s. Specifically, the company was seeking guidance on their ongoing Phase II trial which is being conducted at the University of Pittsburgh Medical Center (Click here to read more about this clinical trial).
The FDA has indicated in a written response to Voyager, that if the Phase II randomised, placebo-controlled trial (involving approximately 42 participants) were to meet its primary endpoint (demonstrating a statistically-significant difference of diary ON-time without troublesome dyskinesias compared to the placebo surgery group) that this may be considered sufficient for acceptance of submission for review of a biologics license application (or BLA).
What is a BLA?
A Biologics License Application (BLA) is a “request for permission to introduce, or deliver for introduction, a biologic product”. To be considered for a BLA, the license application must provide a multidisciplinary FDA review team (which usually includes medical officers, microbiologists, biostatisticians, etc.) with the efficacy and safety information necessary to make a risk/benefit assessment. And during this process, the proposed manufacturing facility of the new treatment will undergo a pre-approval inspection by representatives of the FDA.
Source: Slideshare
With regards to Voyagers questions, the FDA is indicating that if the results of a randomised, placebo controlled Phase II trial are positive that they would be prepared to speed up the process of clinical approval (accepting for review a BLA).
But the FDA also indicated that if the results of the Phase II trial did not achieve the primary endpoint (demonstrating a statistically-significant difference of diary ON-time without troublesome dyskinesias compared to the placebo surgery group), a randomized, placebo-controlled Phase 3 trial involving approximately 120 participants could be used instead for an BLA – if this were to achieve the primary endpoint (in the absence of any major safety concerns).
The results of the Phase II trial are expected to conclude in late 2020/early 2021.
That sounds very positive. What was the second announcement?
Voyager Therapeutics also shared the early findings from a Phase I study evaluating a different route of administration of their treatment VY-AADC. Rather than injecting the treatment from the top of the skull (or transfrontal), this new study was injecting from the posterior (or back) of the head.

Brain surgery. Source Bionews-tx
The purpose of this change was an attempt to improve the spread of infection of the virus in the region of the brain being injected (the putamen). The company wanted to increase the area being infected with VY-AADC, which will hopefully increase the area of the putamen that will now produce the AADC protein.
In addition to positive safety/tolerability results, this new study also showed increased coverage of the putamen in all eight patients (approximately 50% of the putamen was infected with VY-AADC, which was more than delivery from the top of the skull achieved). Another benefit of the new approach was a reduction in surgical times (by two to three hours).
The eight participants in this new study were 57 years of age on average, and had had Parkinson’s for an average of nine years. They were all exhibiting advanced features of PD, and were considered candidates for surgical interventions, such as deep-brain stimulation.
Brain imaging data collected thus far suggests that the increase in AADC enzyme activity in this new trial is consistent with the increases in AADC enzyme activity observed in Cohort 3 (the highest dose group) from the Phase Ib trial (Click here to learn more about those Phase Ib results). And the improvements in motor function (from baseline to six months) across multiple assessments have been consistent with the results from Cohorts 2 and 3 in the Phase Ib trial over the same period of time. The investigators also reported similar reductions in levodopa and equivalent medications to the previous study.
Based on the results of this new study, Voyager has decided that the posterior (back of the head) delivery of VY-AADC will be used in the Phase II (and if required Phase III) clinical trials. In addition, based on all of the Phase I data presented thus far, the company has decided on a treatment dose (up to 900 µL) at concentrations between that used for cohort 2 & 3 in the Phase Ib study (1.5 × 1012 vg in cohort 2 & 4.5×1012 vg in cohort 3).
It all sounds very positive.
So what does it all mean?
Gene therapy biotech company Voyager Therapeutics provided an update this week regarding their clinical trial programme for advanced Parkinson’s. The announcement suggested positive interactions with the regulators regarding the development of the clinical trial programme, as well as interesting new clinical results.
Gene therapy approaches for Parkinson’s have held great promise, but thus far they have struggled to meet that promise. And it is important for readers to remember that what Voyager is proposing is not a curative or neuroprotective treatment.
The VY-AADC product will only allow the brain to produce more dopamine and alleviate the motor features of Parkinson’s. While this treatment may allow individuals to reduce the number of tablets they need to take each day, it will not slow the progression of Parkinson’s. Having said that, VY-AADC may provide a significant improvement in quality of life to those with more advanced Parkinson’s, and in the absence of a curative treatment, an addition treatment options is certainly better than nothing.
Fingers crossed for the on-going Phase II trial.
EDITOR’S NOTE: Voyager Therapeutics is a publicly traded company. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. Voyager Therapeutics has not requested that this material be produced, nor has the author had any contact with the company or any associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from Voyager Therapeutics.







Thank you for this well articulated article Simon. I am not from a medical background, and the point summaries for both the four phases of medical trials, as well as the three types of meetings (A/B/C), really helped me to understand.
LikeLike
Hi Paula,
You’re welcome. Glad you like the content.
Kind regards,
Simon
LikeLike
Thanks!
LikeLike
You’re welcome!
LikeLike
Instead of pills they are going to drill a hole into the brain ! We need something else to go into that hole to stop Parkinson’s . At least ancient peoples put holes in skulls to let the evil spirits out!
LikeLike
Hi Janet,
Thanks for your comment, and you are right: we do need something else to go through those holes to stop PD. Researchers are working on many different versions of things that don’t even need to be injected through holes in the head (for example https://scienceofparkinsons.com/2017/09/16/aav/). Hopefully in the not-too-distant future with just a jab in the arm from one of these engineered viruses (mentioned in the link previous sentence) we’ll be able to help a lot of people with PD to either slow or reverse the condition.
I hope this helps.
Kind regards,
Simon
LikeLike
Pardon me for being a newbie to biology. So this gene is expected to create instructions to let the brain naturally produce Dopamine without ingested oral Levodopa? In other words, is this gene therapy expected to be a one-stop solution for lost neurons, or does it just help with processing oral levodopa better?
LikeLiked by 1 person