|
Parkinson’s is a neurodegenerative condition. This means that cells in the brain are being lost over time. Any ‘cure’ for Parkinson’s is going to require some form of cell replacement therapy – introducing new cells that can replace those that were lost. Cell transplantation represents one approach to cell replacement therapy, and this week we learned that the Japanese regulatory authorities have given the green light for a new cell transplantation clinical trial to take place in Kyoto. This new trial will involve cells derived from induced pluripotent stem cells (or IPS cells). In today’s post we will discuss what induced pluripotent stem cells are, what previous research has been conducted on these cells, and what we know about the new trial. |

Source: Glastone Institute
The man in the image above is Prof Shinya Yamanaka.
He’s a rockstar in the biomedical research community.
Prof Yamanaka is the director of Center for induced Pluripotent Stem Cell Research and Application (CiRA); and a professor at the Institute for Frontier Medical Sciences at Kyoto University.
But more importantly, in 2006 he published a research report that would quite literally ‘change everything’.
In that report, he demonstrated a method by which someone could take a simple skin cell (called a fibroblast), grow it in cell culture for a while, and then re-program it so that it would transform into a stem cell – a cell that is capable of becoming any kind of cell in the body.
The transformed cells were called induced pluripotent stem (IPS) cell – ‘pluripotent’ meaning capable of any fate.
It was an amazing feat that made the hypothetical idea of ‘personalised medicine’ suddenly very possible – take skin cells from anyone with a particular medical condition, turn them into whatever cell type you like, and then either test drugs on those cells or transplant them back into their body (replacing the cells that have been lost due to the medical condition).
Personalised medicine with IPS cells. Source: Bodyhacks
IPS cells are now being used all over the world, for all kinds of biomedical research. And many research groups are rushing to bring IPS cell-based therapies to the clinic in the hope of providing the long sort-after dream of personalised medicine.
This week the Parkinson’s community received word that the Pharmaceuticals and Medical Devices Agency (PMDA) – the Japanese regulatory agency that oversees clinical trials – have agreed for researchers at Kyoto University to conduct a cell transplantation trial for Parkinson’s, using dopamine neurons derived from IPS cells. And the researchers are planning to begin their study in the next month.
In today’s post we are going to discuss this exciting development, but we should probably start at the beginning with the obvious question:
What exactly is an IPS cell?
In 2006, this study was published:

Title: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors.
Authors: Takahashi K, Yamanaka S.
Journal: Cell. 2006 Aug 25;126(4):663-76.
PMID: 16904174 (This article is OPEN ACCESS if you would like to read it)
In this study, Shinya Yamanaka‘s team started with the hypothesis that proteins which are important to the maintenance of embryonic stem cells (the cells that give rise to all of the cells in your body) might also be able to cause an embryonic state in mature adult cells. They selected twenty-four proteins that had been previously identified as important in embryonic stem cells to test this idea. They used re-engineered viruses to deliver these proteins to mouse skin cells. The viruses were emptied of all their disease causing properties, and could thus function as very efficient biological delivery systems.
The skin cells were genetically engineered in such a fashion so that only cells in which reactivation of the embryonic stem cells-associated protein, Fbx15, would survive the testing process. If Fbx15 was not turned on in the cells, over time they would die. When the researchers infected the cells with all twenty-four embryonic stem cells genes, remarkably some of the cells actually survived and began to divide like stem cells.
In order to identify which proteins were necessary for the reprogramming, the researchers began removing one protein at a time from the pool of twenty-four. Through this process, they were able to narrow down the most effective proteins to just four: Oct4, Sox2, cMyc, and Klf4, which became known as the Yamanaka factors.
This new type of cell is called an induced pluripotent stem (IPS) cell.
And in acknowledgement of this amazing bit of research, in 2012 Prof Yamanaka and Prof John Gurdon (University of Cambridge) were awarded the Nobel prize for Physiology and Medicine for the discovery that mature cells can be converted back to stem cells.

Prof Yamanaka and Prof Gurdon. Source: UCSF
Prof Gurdon achieved a similar feat to Yamanaka’s work in 1962 when he removed the nucleus of a fertilised frog egg cell and replaced it with the nucleus of a cell taken from a tadpole’s intestine. That is to say, the nucleus of a mature cell was placed into a frog egg cell, and guess what happened? The modified egg cell then grew into an adult frog! This fascinating research proved that the mature cell still contained the genetic information needed to form all types of cells.
EDITOR’S NOTE: I do not want to be accused of taking anything away from Prof Gurdon’s contribution to this field (which was great!) by not mentioning his efforts here. For the sake of saving time and space, we are focusing on Prof Yamanaka’s research as it is more directly related to today’s post.

Making IPS cells. Source: learn.genetics
As I suggested above, the amazing discovery of IPS cells has opened new doors for biological research and provided us with incredible opportunities for therapeutic treatments. For example, we can now take skins cells from a person with Parkinson’s and turn those cells into dopamine neurons which can then be tested with various drugs to see which treatment is most effective for that particular person (personalised medicine in it’s purest form).

Some of the option available to Parkinson’s disease. Source: Nature
Some of those dopamine neurons could also potentially be transplanted back into the person – into the brain to replace the lost dopamine neurons. This process would hopefully reduce the need for medication that suppresses the immune system, because the body would recognise the cells being transplanted as ‘self’ (or derived from the person being transplanted).
Imagination is literally the only limiting factor with regards to the possible uses of IPS cell technology.
How do IPS cells differ from embryonic stem cells?
This is very simple:
IPS cells are engineered in a laboratory, while embryonic stem cells are a natural form of pluripotent stem cell – derived from a very early stage embryo.

Embryonic stem cells in a petridish. Source: Wikipedia
The process of collecting embryonic stem cells begins with a fertilised female egg cell. The egg cell will divide, to become two cells, then four, eight, sixteen, etc. Gradually, it enters a stage called the ‘blastocyst’, which is a small ball of cells. Inside the blastocyst is a group of cell that are called the ‘inner stem cell mass’, and it is these cells that can be collected and used as embryonic stem cells.

The process of attaining embryonic stem cells. Source: Howstuffworks
Although they differ in their origins, IPS cell and embryonic stem cells both share many similarities, such as the ability to become any cell inside your body.
Are there other research groups using embryonic stem cells for cell transplantation in Parkinson’s?
Yes, there are, and we recently discussed and summarised some of those efforts in another post (Click here to read that post). In this post we are going to focus on the Kyoto research team.
So who are the Kyoto research team?
The Kyoto research team is led by Prof Jun Takahashi:
Prof Jun Takahashi. Source: gforce-pd
He is the Head of Department of Clinical Applications at the Center for iPS Cell Research and Application, Kyoto University
He and his research team have a long history of working with IPS cells, particularly in the context of models of Parkinson’s:
Title: Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson’s disease.
Authors: Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, Saiki H, Miyamoto S, Takahashi J.
Journal: J Parkinsons Dis. 2011;1(4):395-412. doi: 10.3233/JPD-2011-11070.
PMID: 23933658
This study was one of the first investigations of IPS-derived dopamine neurons being transplanted into both a mouse model of Parkinson’s and a primate model of Parkinson’s. The researchers found that the dopamine neurons survived in a monkey brain for at least six months (the length of the study).
More recently, the Kyoto team have been trying to better purify the population of cells being transplanted:
Title: Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1
Authors: Samata B, Doi D, Nishimura K, Kikuchi T, Watanabe A, Sakamoto Y, Kakuta J, Ono Y, Takahashi J.
Journal: Nat Commun. 2016 Oct 14;7:13097.
PMID: 27739432 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers utilised a double selection strategy for identifying the cells to transplant into both a rodent model of Parkinson’s and (again) a primate model of Parkinson’s. The transplants resulted in a significant improvements in motor behaviour in the animals, without any signs of tumor formation.
And the researchers have also investigated other approaches to cell selection (Click here, here, here and here to read more about this).
These efforts all lead to a long-term study of transplanted IPS-derived dopamine neurons in a primate model of Parkinson’s:
Title: Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model.
Authors: Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, Mizuma H, Takara S, Takahashi R, Inoue H, Morita S, Yamamoto M, Okita K, Nakagawa M, Parmar M, Takahashi J.
Journal: Nature. 2017 Aug 30;548(7669):592-596.
PMID: 28858313
In this study, the researchers transplanted human IPS cell-derived dopamine neurons into a primate model of Parkinson’s and then followed some of the animals over 2 years. Not only did the transplanted cells help to restore normal function in these animals, but there was no sign of any tumors. In this study (and others – click here for an example), the Kyoto team focused a lot of effort on limiting the immune system response to the transplanted cells, by carefully selecting the cells to be transplanted (more on this below).
And Prof Takahashi’s research team has also spent a lot of time making IPS cells from people with Parkinson’s and testing their ability to be transplanted:
Title: Idiopathic Parkinson’s disease patient-derived induced pluripotent stem cells function as midbrain dopaminergic neurons in rodent brains.
Authors: Kikuchi T, Morizane A, Doi D, Okita K, Nakagawa M, Yamakado H, Inoue H, Takahashi R, Takahashi J.
Journal: J Neurosci Res. 2017 Sep;95(9):1829-1837.
PMID: 28233934
Interestingly, in this study, the researchers found that there was no significant vulnerability between dopamine neurons generated from IPS cells from people with idiopathic (or spontaneous) Parkinson’s compared to IPS cells from healthy controls. Both sets of cells survived and functioned in a rodent model of Parkinson’s, as well as a genetically engineered mouse which produces too much Parkinson’s-associated alpha synuclein.
Needless to say, Prof Takahashi and his team have a great deal of experience transplanting dopamine neurons derived from IPS cells into models of Parkinson’s.
And they are now keen (and confident) to take this method into clinical testing.
So what do we know about the new trial?
Prof Jun Takahashi (speaking) and colleagues. Source: Japantimes
Information provided by Kyoto University (Click here for the study website) and the Japanese media has indicated that this Phase I/II clinical trial aims to investigate the safety and efficacy of transplanting human IPS cell-derived dopaminergic progenitors into the brains of people with Parkinson’s (what stage of PD has not been disclosed). The study will involve just 7 participants, and it will be starting very soon (recruitment began the day of the announcement).
Physicians at Kyoto University Hospital will drill a 12-mm (0.5-inch) hole into the skull of the participants and 5 million of ‘dopaminergic precursor cells’ (derived from IPS cells) will be injected into a region of the brain called the putamen. This is one of the main regions of the brain where the dopamine neurons release their dopamine. The image below demonstrates the loss of dopamine (the dark staining) over time as a result of Parkinson’s (PLEASE NOTE that the time scale presented here varies from person to person):
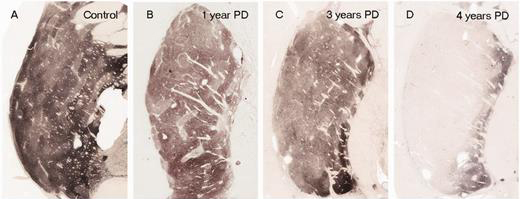
The loss of dopamine in the putamen as Parkinson’s progresses. Source: Brain
In cell transplant procedures for Parkinson’s, multiple injections are usually made in the putamen, allowing for deposits in different areas of the structure. These multiple sites allow for the transplanted cells to produce dopamine in the entire extent of the putamen. And ideally, the cells should remain localised to the putamen, so that they are not producing dopamine in areas of the brain where it is not desired (possibly leading to side effects).

Targeting transplants into the putamen. Source: Intechopen
One important feature of this new IPS cell clinical trial is that rather than making patient-specific IPS cells (that is IPS cell unique and specific to each patient), the IPS cells being used in the trial are being “created using cells from people who have types of immunity that make them less prone to transplant rejections” (Source).
Several years ago, Kyoto University launched the “Stock Project”. This was an initiative to generate IPS cells that could be transplanted into a lot of people without generating an immune response. It was estimated that just 50 lines of carefully selected IPS cells would cover 73% of the Japanese population by the matching of three types of immune system markers (HLA-A, B, and DR – Click here to read more about this).
Presumably for this first study (I am speculating here for the purpose of discussion) the Kyoto team have generated IPS cells that have little or no human leukocyte antigen (HLA). HLA is a protein that is present on the surface of cells, and it is recognized by the cells of the immune system (such as T-lymphocytes). Proteins like HLA play a crucial role in the immune response after the transplantation of foreign (‘not self’) cells. Using IPS cells with no HLA will hopefully make it less likely that the cells will be rejected by the immune system, giving the cells a better chance of surviving long term.
In addition to using ‘immune system safe’ cells, the patients will also be treated with a drug called tacrolimus which is a drug that suppresses that immune system, dampening down any immune response and giving the cells the best chance of success.
The participants in the study will be followed and assessed for 2 years post transplantation.
The researchers are aiming to develop the method as a new treatment that will be covered by national health insurance program in Japan, making it available to a large number of people affected by Parkinson’s. This is a noble gesture, one that possibly also acknowledges the enormous investment that the Japanese nation has made in the IPS technology.
How can I get involved with the trial?
The study is only open to people who live in Japan and are covered by the Japanese health insurance scheme.
The University of Kyoto has set up a recruitment page for the trial (Click here to see that page), but some translation will be required.
Are future trials planned?
This is unclear at present and really depends on the outcome of this first trial. And the Japanese will be proceeding cautiously for reasons we will discuss shortly.
This is only the third human clinical trial using IPS cells approved in Japan. The first was launched in 2014, using retinal cells derived from IPS cells – the goal was to replace eye tissue damaged by a condition called age-related macular degeneration (or AMD). That study was led by Prof Masayo Takahashi of the RIKEN Center for Developmental Biology in Kobe – who just happens to be Prof Jun Takahashi’s wife.
Prof Masayo Takahashi. Source: Youtube
So you can see there is a great deal of IPS cell experience involved with this new trial.
While the AMD treatment was initially reported to be safe, there was one reported adverse event (Click here to read more about this). A report of that study was published last year (Click here to read the report).
The second IPS cell clinical trial approved by the Japanese regulators was only given the green light earlier this year. A team at Osaka University in Japan will be conducting an IPS cell–based clinical trial for ischemic heart disease.
Why would the Japanese be proceeding cautiously?
It should be noted that all of the research efforts taking stem cell based cell transplantation to the clinic are proceeding cautiously, because of the worry that just a few cells in the transplanted population will not turn into dopamine neurons, but rather something completely different. Given their pluripotent potential, this is an issue for both IPS and embryonic stem cell approaches to cell transplantation. And once something is transplanted into the brain it is very difficult to ‘un-transplant’ it.
Older cell transplantation methods, such as fetal tissue-based approaches do not have this problem, because the transplanted tissue is being dissected from neural tissue from embryos – those cells are all committed to being brain cells. But that approach has ethical issues as well as tissue supply problems.
Some readers may point out that an added worry with IPS cells is that the process of transforming the cells from skin cells to IPS cells can leave the transformed cells with random genetic mutations. This is probably less of a concern, however, because the Japanese have been screening all of their IPS cell lines for any cancer-associated genetic mutations, and rejecting anything that looks suspicious.
The Kyoto team are certainly very confident that their process of maturating and transplanting the IPS-derived cells is ready for going to the clinic. As we discussed above they have a great deal of experience with the technology, and they have not observed any tumors (or other oddities) in any of their preclinical transplantation results (out to 2 years post-transplantation), suggesting that the cells are safe to use.
Despite all of this, it is probably still wise to be cautious and prudent.
So what does it all mean?
In January, in our ‘Expectations for the year ahead’ post (Click here to read that post), one of the big expectations was a lot of clinical research activity kicking off around cell transplantation for Parkinson’s. And we have not been disappointed. Recently, Chinese researchers have announced the start of their clinical trial (Click here to read more about that), this week we have heard that the team in Kyoto is going to be starting in the next month, and in coming months we hope to hear about the commencement of a US-based clinical study being conducted by a biotech company called BlueRock Therapeutics.
In addition, there is the on going Transeuro study testing the fetal transplantation approach for Parkinson’s.
The Transeuro trial. Source: Transeuro
And there is a cell transplantation clinical study being conducted in Melbourne (Australia), by an American company called International Stem Cell Corporation (ISCO).

So all-in-all, there is a great deal of research activity on the cell replacement therapy front. And this is very encouraging to see after a long period of high hopes for this therapeutic approach.
Here at the SoPD, we will be keeping a close eye on the activities in Kyoto, and be listening in for any news when it becomes available. And we certainly wish the research team the best of luck with their new study.
Oh, and of course the IPS cell trial is not the only big thing happening in Kyoto! Next year is the 5th World Parkinson Congress will be held on the 4th-7th June 2019.
It will be a fantastic week of research presentations from world leading scientists, networking with members of the Parkinson’s community from all over the world, and raising awareness on a global scale about this debilitating condition.
And yours truly is planning to be there.
EDITORIAL NOTE #1 – It is important for all readers of this post to appreciate that cell transplantation for Parkinson’s is still experimental. Anyone declaring otherwise (or selling a procedure based on this approach) should not be trusted. While we appreciate the desperate desire of the Parkinson’s community to treat this condition ‘by any means possible’, bad or poor outcomes at the clinical trial stage for this technology could have serious consequences for the individuals receiving the procedure and negative ramifications for all future research in the cell transplantation field.
EDITORIAL NOTE #2 – the author of this blog is associated with research groups conducting the current Transeuro trial. He has endeavoured to present an unbiased coverage of the news surrounding the current clinical trials. Any opinions offered here are solely his own and do not represent those of any associated parties. He had not discussed the recent news with any colleagues before publishing this post.
The banner for today’s post was sourced from toursbylocals.











Wow….just Wow. Thank you, Simon
LikeLike
Hi Ruth,
You are very welcome. Glad you liked the post.
Kind regards,
Simon
LikeLike
great summary!
many thanks
LikeLike
Glad you liked it Kevin. You’re very welcome.
Kind regards,
Simon
LikeLike
Hi Simon !
Thank you so much !!
Can you write and analyse also (later, when it’s fit with your timeschedule) about something “almost” the same who is going on
in Amerika (Prof. Jeanne Loring) ??
Greetings,
Jan
LikeLike
Hi Jan,
Thanks for the comment. Ben Stecher interviewed Prof. Loring last year (https://tmrwedition.com/2017/11/16/q-a-with-stem-cell-pioneer-prof-jeanne-loring/) and she suggested that they are looking at 2019 before they will be starting any clinical trials for their cell work. When that trial kicks off, you can expect a post here.
Kind regards,
Simon
LikeLike
This is one of the most well researched articles on PD i’ve ever stumbled across. If one chooses to go through all the reference material that’s linked in the various sections, one’s knowledge of the subject is bound to increase manifold.
Wishing Prof. Takahashi and his colleagues at Kyoto University all the very best with the first phase of the trial. Wish you team as well as the other making the attempt all the best as well!
Let’s cure this!
LikeLike
Hi Aaychat,
Thanks for the comment – glad you liked the post. Fingers (and toes) are crossed for the trial.
Kind regards,
Simon
LikeLike
I wonder if you could comment on the differences between these transplanted neurons and the brain’s own dopamine neurons.
For example, your report says that the exogenous neurons are injected into the putamen, while endogenous dopamine neurons have their cell bodies in the substantial nigra and project their axons into the putamen and the caudate nucleus, where their axon terminals produce dopamine that is released in response to the specific firings of those neurons (which originate from their cell bodies, presumably under some kind of meaningful control based on the immediate need for more dopamine).
So having these neurons injected into the putamen would seem mainly to increase the overall level of dopamine available there. Aside from possibly providing a more stable release of dopamine, how is that different in its effects from a steady source of exogenous levodopa (e.g. from a Duopa pump)?
My understanding is that levodopa is processed into dopamine by HT5 (serotonin) neurons, and released near dopamine neurons’ axon terminals for reuptake, so how are these transplanted neurons going to do better than that?
LikeLike
Hi Lou,
Great question. Previous preclinical efforts to transplant dopamine neurons into the substantia nigra of the adult rodent brain found that the axons from the transplanted cells struggled to reach all the way to the striatum and didn’t really innervate that target region as hoped. This finding resulted in the idea that there are inhibitory signals in the adult brain that are limiting the guidance and branching of axons. In addition, the substantia nigra is a very deep and difficult target region in humans – and given the important functions of the brain stem, neurosurgeons are reluctant to disrupt it too much.
Thus, targeting the cell transplants to the putamen became the next best thing. Particularly as transplanting to this site isolates the generation of dopamine to where it is mostly needed. L-dopa treatment – tablet or pump based – results in high levels of dopamine being produced in many different regions. Yes, 5HT (Serotonin) cells can generate dopamine from L-dopa, but they do not have the right packaging for it and they appear to release it in a haphazard fashion, rather than the coordinated approach dopamine neurons use. Plus 5HT cells do not have the dopamine transporter – which reabsorbs excess dopamine after it has done its job. This results in extra dopamine floating around in the brain, which has lead some researchers to ask if 5HT cells could be involved with L-dopa induced dyskinesias. In addition, 5HT cells have axons reaching to different areas of the brain compared to dopamine neurons. So you can start to see why it is important to transplant the right cells into the right region. Ideally, the transplanted neurons will largely be dopamine neurons, and previous research suggests that the transplanted dopamine neurons have all the appropriate biological machinery (eg dopamine transporter, etc) as the endogenous dopamine neurons.
Other recommended reading on the topic of cell transplantation for Parkinson’s:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3250287/
http://dev.biologists.org/content/145/1/dev156117.long
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4232736/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5399709/
I hope all this helps.
Kind regards,
Simon
LikeLike
I had been hopefully skeptical regarding the prospects of such transplantion, but now, after reading your very illuminating response, I think I may be skeptically hopeful. This is truly exciting and creative work. I only wish that we had a health care system in the U.S. that would do something like the Japanese system is doing, by planning to provide this treatment to every PD patient in the nation. Maybe your British NHS would still be capable of such a sincere act of caring for a nation’s population.
I have to marvel at how much of nature’s capacities during gestation and infancy can be re-invoked in adulthood for the purpose of replacing damaged cells. With a little tweaking, these iPSCs not only know how to differentiate into dopamine neurons, but apparently have some idea of how to hook themselves up to their surroundings when they do.
As you’ve pointed out, there are limits to this capacity, at least for the present, when trying to get neurons implanted in the SN region to project to the striatum. But I see from the articles linked in your reply that neurons transplanted to the putamen *have* been shown to be capable of branching out multiple axon terminals, and then synapsing these onto existing dopamine receptors (or else forming entirely new ones, I would suppose opposite pre- and post-synaptic receptors on glutamatergic and other neurons).
It’s astonishing to me that these neurons seemingly know which other neurons to connect to when they land on the foreign shores of a previously-unknown (to them) area of the brain. That kind of specificity is certainly far beyond any existing surgical techniques, so getting the body to use its own wisdom to make those connections is perhaps the *only* way that one could imagine this kind of repair process as possible.
One thing I’m still wondering is: by what process are these implanted neurons stimulated to fire? I mean, there must be certain stimuli that an endogenous DA neuron’s cell body receives in the SN that stimulate it to fire, but are these same stimuli (e.g., other neurons synapsing onto the DA neurons’ dendrites) available to an implanted DA neuron whose cell body is in the putamen? Because if these neurons are not just leaking out dopamine from imperfect vesicles, as you’ve described the HT5 neurons as doing under levodopa therapy, then it seems that they would have to fire in order to release their dopamine.
LikeLike
Also, there’s a disease process at work in PD that involves some kind of autoimmune feedback loop (neurons dying, alpha synuclein and neuromelanin being released from the damaged neurons, microglia getting triggered by those substances and by fibrils and plaques formed from alpha synuclein, causing them to generate cytokines that attack neurons in the area, causing more neurons to die, and so on). So, I’m wondering whether these exogenous neurons would be more or less likely to become affected by that disease process. Is it anticipated that they will eventually die through the same process as other neurons die in PD, and thus will periodically need to be replenished?
LikeLike
Hi Lou,
Another great question. And the available research suggests that the transplanted cells can get caught up in the disease process. There is cases of transplanted dopamine neurons exhibiting Lewy bodies for example. Only a small number of the transplanted cells, but the transplanted cells do appear to be vulnerable. A recent study of a postmortem analysis of a Parkinson’s brain 24 year after transplantation found that 10 – 12% of the transplanted dopamine neurons had Lewy bodies (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4988567/). Previous report of 8-10 year old transplants had found much lower percentages of Lewy bodies (1-2% in this study: https://www.ncbi.nlm.nih.gov/pubmed/18391963).
Despite this finding, the data doesn’t suggest that the cells are ‘more likely to be affected by the disease’. Perhaps as younger dopamine neurons, they are more robust. Plus, the patients in these studies are usually on immunosuppression medication for long periods which results in less chance of immune rejection. So if the cells survive the transplantation procedure, there should not need to be a periodic replacement required. I hope this answers your question.
Kind regards,
Simon
LikeLike
Beyond the relative youth of these transplanted neurons, I wonder whether their being implanted to the putamen rather than to the SN also gives them a better chance of survival, given that the disease process might be more active in the SN area, where all the diseased endogenous cell bodies are located. I’m imagining that alpha-synuclein and neuromelanin are getting released to the inter-cellular environment within the SN each time a DA neuron there is damaged and/or dies, so perhaps those two substances are much more highly concentrated (and in the case of AS, aggregated) there. The putamen might therefore be a more favorable environment for the new transplants, increasing their chances of surviving in good condition.
LikeLike
Hi Lou,
It is a very good point, but the main reasons are simply ease of access (compared to targeting the midbrain) and that the putamen is the target release point for the dopamine. Insightful observation though.
Kind regards,
Simon
LikeLike
Really off topic but maybe a useful idea – is there a place in your blog where you summarize the five or so most important studies we are currently waiting for results on – that will be coming in this year or early next year? Kind of a “Science of Parkinson’s for dummies” post I guess. I get lost in all the information sometimes – even though it is really presented well. Seems like you have covered this ground a little in the past – but I am not remembering where. Thanks
What would I do with this information? Chew my fingernails from anxiey, increase my hope and thus my dopamine level, and become even more addicted to your blog I think.
LikeLike
Hi DKDC,
I hope all is well. You raise an interesting point that I have been contemplating for a while – is the site full of too much information? Not seeing the forest for the trees – sort of thing. How to better present it? I wish I had a free moment to try and address this, but free time is a luxury at present. The ‘Road ahead’ post from the start of the year is probably the best option for what we are looking out for this year and next (https://scienceofparkinsons.com/2018/01/07/2018/) – the ambroxol clinical trial results is what I am currently waiting for.
I am happy to hear ideas regarding how better to present things (as long as it doesn’t require more work!).
Kind regards,
Simon
LikeLike
While we wait, what can we do through exercise, fasting, etc., to “activate” stem cells within our own brains, if such a process exists?
LikeLike
Hi Paul,
Thanks for your comment.
Current research suggests that exercise and fasting do seem to help cells function better (particularly with regards to waste disposal (or autophagy) in the case of fasting), and may help to stimulate stem cells to divide, but those newly produced cells do not result in the replacement of the lost dopamine neurons. Many of the new cells become astrocytes (or helper cells). Hence the need for cell replacement approaches like cell transplantation or cell reprogramming (https://scienceofparkinsons.com/2017/04/18/on-astrocytes-and-neurons-reprogramming-for-parkinsons/).
I hope this answers your question.
Kind regards,
Simon
LikeLike
So there is some benefit in fasting – like there is in sleeping to keep the brain cleaned out and thus possibly help keep the progression slower than if we stayed up all night eating ice cream? Thanks
LikeLike
Or stay up all night writing blog post post. Yes, DKDC good sleep is important and high dairy diet may not be such a wise idea. We will look at intermittent fasting in a future post (it is on the list of posts to write).
Kind regards,
Simon
LikeLike
When I read your account of how the drug tacrolimus will be used to prevent rejection of implanted DA neurons, I wondered if that drug might be used by itself in calming the autoimmune feedback loop that fuels PD progression. Upon doing a little search for that, I found that trials of such a therapy are already being planned.
Given that tacrolimus is being considered as a therapy for PD itself, treating patients with that drug may make it difficult to ascertain whether the source of any improvements noticed come from the transplanted cells, or from the tacrolimus. Maybe those two efforts can coordinate so that the tacrolimus effort could serve as kind of a control group for the tacrolimus + implantation study. Given how methodical these scientists are, they’ve probably already thought of that.
LikeLike
Hi Lou,
I know that there are efforts afoot to test Tacrolimus as a monotherapy for PD. If that project ever comes to fruition, we will discuss it further here on the SoPD. Until then, you are right that the potential effects of the immunosuppressant and cell transplants will be difficult to differentiate. And until we get more information regarding the Japanese trial, we’ll have to wait and see how they are planning to deal with it.
Kind regards,
Simon
LikeLike
It looks like this therapy is still a few years away from becoming generally available, even in Japan. I’m a caregiver for a partner is who doing pretty well considering that she is 70 and in her 12th year since diagnosis (about at Hoehn and Yahr level 2.5, by my own rough estimate). So I’m wondering what kinds of criteria might determine eligibility for this procedure, and what might be done to try to ensure that she’s in a condition that could still benefit from it by the time it becomes more generally available.
LikeLike
Hi Lou,
This is a really interesting question.
One of the exclusionaries for all of the transplantation trials is dyskinesias. There are serious concerns in the research community that adding dopamine cells to such a situation could do more harm than good. In two previous cell transplantation trials, some of the participants in the studies developed graft-induced dyskinesias – that is to say, the transplanted cells caused the dyskinesias.
Such information regarding exclusionaries should not be a disappointment for individuals already affected by dyskinesias. A lot of folks in the community put a great deal of hope on cell replacement therapy, but it must be understood that it is not a ‘curative’ treatment – the condition is still progressing. I myself am more focused on the disease halting approaches. Once that nut is cracked, all options are on the table for improving quality of life for people. And in some cases, cell replacement therapy may not be the best option compared to something more ‘programmable’, like deep brain stimulation. And I will admit that I have gradually become more inclined towards DBS simply because the cell transplant approach takes so long to show any real benefits (once transplanted the immature cells take 2-3 years before they are mature enough to have major impact). DBS, on the other hand is more immediate. Perhaps these engineered iPS cells will be different. Again we’ll have to wait and see.
I hope your partner is doing well.
Kind regards,
Simon
LikeLike
No dyskinesias to speak of so far, so, fingers crossed, then.
Like you, beyond the essential palliative therapy, we’ve been focused mainly on slowing disease progression, simply because there is no way (yet) to replace neurons once they are lost, so hanging onto original equipment would seem to be priority #1. Specifically, we are intent on disrupting the innate immunity autoimmune feedback that loops from neuron damage to released alpha synuclein and neuromelanin, to microglia whose receptors are activated by those two substances, to cytokines released by those microglia, and back to damage of bystander neurons, perpetuating the cycle.
To do this, we use a variety of means that affect the autoimmune loop a various points. We support mitochondrial function with ubiquinol (and soon, per your article, with TUDCA). We inhibit AS aggregation with Longvida curcumin, and inhibit microglia receptors with that same curcumin plus baicalin and EGCg. We inhibit the effects of cytokines released by activated microglia with NAC, ALC and ALA. There has still been progression, but a rough estimate says she is doing better than about 70 percent of patients (according to the one study I’ve found on the average rate of progression).
If you’re interested, the approach we’re using is written up here, with links to pertinent research:
https://www.smartpatients.com/conversations/41861
The term “cure” is tricky when the typical patient has already lost the majority of DA neurons by the time of diagnosis. Sure, stopping progression would halt the disease process, but it wouldn’t greatly improve whatever the symptoms were at that point. Whereas, transplantation *can* improve them, even if progression continues. Fortunately, it’s possible to pursue both approaches.
LikeLike
Are you skeptical about cell replacement therapy working? Does deep brain stimulation help with motor symptoms even in the long run to the point that motor symptoms can be dealt with effectively even in old age?
Where do we stand from a monoclonal antibody/ disease halting standpoint? Do the initial results look promising
LikeLike
Hi Deepak,
Thanks for your comment. I am not skeptical about cell replacement therapy working. The issue for me is more that the transplanted cells take so long to mature and to start producing dopamine (2-3 years). DBS provides a more immediate result.
The monoclonal antibody approach is still being tested and I don’t like to speculate regarding ongoing clinical trials. The results thus far have indicated that the treatment is very safe, which is encouraging, and we should be getting the first hints next year as to whether the treatment is having any effect.
Kind regards,
Simon
LikeLike
I felt like DBS was for people whose response to levodopa was poor and they developed dyskinesias. More for late stage disease. Cell replacement would be for early to mid stage patients where LDOPA response is still good, and cell replacement would be a more physiological release of DA without any oral meds? If I’m not wrong they don’t even treat the same disease stage
LikeLike