|
The clustering (or aggregation) of the protein, alpha synuclein, is a cardinal feature of the Parkinsonian brain, and it is believed to be associated with the neurodegeneration that characterises the condition. As a result, many pharmaceutical and biotech companies are focused a great deal of attention on identifying novel compounds that can enter the brain and inhibit alpha synuclein from aggregating. Recently, a collaboration of companies published the results of an amazingly large study highlighting novel inhibitors. But an interesting aspect of the results was the ‘positive control’ compound they used: Epigallocatechin Gallate (or simply EGCG) In today’s post, we will review the results of the study, discuss what EGCG is, and look at what is known about this compound in the context of Parkinson’s.
|
Source: Cargocollective
Every now and then, the research report of a huge study comes along.
And by that, I don’t mean that the results have a major impact. Rather, I am referring to the scope and scale of the work effort required to conduct the study. For example, the GIANT study which is looking for genetic variations associated with height (Click here to read a previous SoPD post that briefly touches on that study).
Recently, the report of one huge study was published:
Title: Potent α-Synuclein Aggregation Inhibitors, Identified by High-Throughput Screening, Mainly Target the Monomeric State
Authors: Kurnik M, Sahin C, Andersen CB, Lorenzen N, Giehm L, Mohammad-Beigi H, Jessen CM, Pedersen JS, Christiansen G, Petersen SV, Staal R, Krishnamurthy G, Pitts K, Reinhart PH, Mulder FAA, Mente S, Hirst WD, Otzen DE.
Journal: Cell Chem Biol. 2018 Aug 29. pii: S2451-9456(18)30271-X.
PMID: 30197194
In this study, researchers from Arrhus University, Biogen, Amgen, Genentech, Forma Therapeutics, & Alentis Pharma screened almost 750,000 different compounds for their ability to interact with the Parkinsons-associated protein alpha synuclein.
And before we go any further, just take a moment to fully appreciate the size of that number again:
Source: peopleforbikes
That is eye watering stuff! That is a “I need to sit down for a moment and let this sink in” kind of number. That is a “Are there that many compounds in all of the known universe?” number.
After reading the number, I was left wondering what each of the scientists involved in this study must have been thinking when the boss first said “Hey guys, let’s screen half a million compounds…. no, wait, better yet, why stop there. Let’s make it 3/4 of a million compounds”
How enthusiastic was the “Yes boss” response, I wonder?
All kidding aside, this is an amazing study (and the actual number of compounds screened was only 746,000).
And the researchers who conducted the study should be congratulated on their achievement, as the results of their study may have a profound impact in the longer-term for the Parkinson’s community – you see, the researchers found 58 compounds that markedly inhibited the aggregation of alpha synuclein, as well as another 100 compounds that actually increased its aggregation. A great deal of research will result from this single, remarkable piece of work.
But of particular interest to us here at the SoPD, was the activity of one of the positive control compounds that the researchers used in some of the tests.
What was the control compound?
In 2006, this research paper was published:

Title: Small molecule inhibitors of alpha-synuclein filament assembly
Authors: Masuda M, Suzuki N, Taniguchi S, Oikawa T, Nonaka T, Iwatsubo T, Hisanaga S, Goedert M, Hasegawa M.
Journal: Biochemistry. 2006 May 16;45(19):6085-94.
PMID: 16681381
In this study, the researchers tested 79 different chemical compounds for their ability to inhibit the aggregation of alpha-synuclein. They found several compounds of interest, but one of them in particular stood out: EGCG.
The researchers found that EGCG robustly blocked the clustering of alpha synuclein.
And it was EGCG that was used as the positive control in the ‘750,000 compound study’ mentioned above.
In fact, when discussing their 58 most potent inhibitors of alpha synclein aggregation, the ‘750,000 compound study’ researchers wrote that they were “comparable with the most promising known fibrillation inhibitors (e.g., baicalein and EGCG) in their ability to suppress” aggregation (We shall be discussing baicalein in a separate up-coming SoPD post – another very interesting compound).
So what is EGCG?

The chemical structure of EGCG. Source: GooglePatents
Epigallocatechin Gallate (or EGCG) is a powerful catechin.
What is a catechin?
Catechins are a group of compounds that are found in tea, fruit, chocolate and wine. They belong to a family of nutrients called flavonoids (a large class of plant pigments), and have been linked to a variety of health benefits.
Importantly, catechins (particularly EGCG) are potent antioxidants.
And what are antioxidants?
Antioxidants are substances which limit oxidation.
And yes, I know what you are going to ask next: What is oxidation?
Oxidation is the loss of electrons from a molecule, which in turn destabilises that particular molecule. Think of iron rusting. Rust is the oxidation of iron – in the presence of oxygen and water, iron molecules will lose electrons over time. Given enough time, this results in the complete break down of objects made of iron.

Rusting iron. Source: Thoughtco
The exact same thing happens in biology. Molecules in your body are constantly going through a similar process of oxidation – losing electrons and becoming unstable. This chemical reaction leads to the production of what we call free radicals, which can then go on to damage cells.
What is a free radical?
A free radical is an unstable molecule – unstable because they are missing electrons. They react quickly with other molecules, trying to capture the needed electron to re-gain stability. Free radicals will literally attack the nearest stable molecule, stealing an electron. This leads to the “attacked” molecule becoming a free radical itself, and thus a chain reaction is started. Inside a living cell, this situation can cause terrible damage, ultimately killing the cell itself.
Antioxidants are the good guys in this situation.
They are molecules that neutralise free radicals by donating one of their own electrons. The antioxidant don’t become free radicals by donating an electron because by their very nature they are stable with or without that extra electron.

How free radicals and antioxidants work. Source: h2miraclewater
So EGCG has antioxidant properties?
Exactly. And in addition to that, as we mentioned above, EGCG was also a potent inhibitor of aggregating alpha synuclein.
What is meant by ‘aggregating alpha synuclein’?
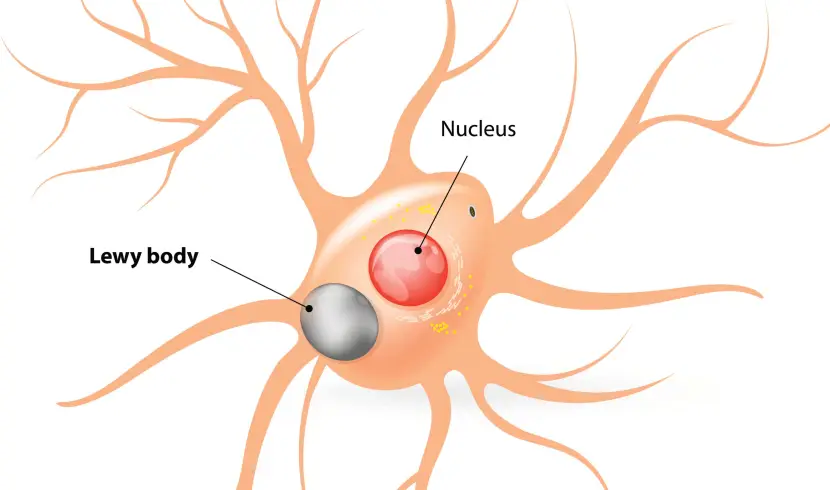
In the Parkinsonian brain, a protein called alpha synuclein clumps (or aggregates) together, which is believed to lead to the appearance of Lewy bodies.
What are Lewy bodies?
Lewy bodies are dense circular clusters of alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies. In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells. In the image below, alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.

Examples of Lewy neurites (stained in brown; indicated by arrows). Source: Wikimedia
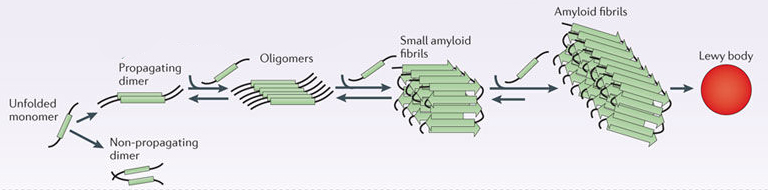
When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure. When it is first produced, alpha synuclein will look something like this:
Alpha synuclein. Source: Wikipedia
In this form, alpha synuclein is considered a monomer – which is a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.

Microscopic images of Monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein give rise to the aggregations of protein that go on to form the Lewy bodies we mentioned above:
Parkinson’s associated alpha synuclein. Source: Nature
Now, given this process – and its association with a neurodegenerative condition like Parkinson’s – you will appreciate that a lot of effort is being put into reducing this aggregation of alpha synuclein protein. It is hoped that by limiting this activity, we may be able to slow or stop completely the progression of the condition.
Hence, the ‘750,000 compound study’ mentioned at the top of this post (in which 5 biotech companies participated).
So EGCG is an antioxidant that also inhibits alpha synuclein aggregation?
Exactly: In addition to having antioxidant properties, EGCG is also remarkably good at blocking the production of alpha synuclein aggregates.
And there have been many studies that have demonstrated this effect:
Title: EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers.
Authors: Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE
Journal: Nat Struct Mol Biol. 2008 Jun; 15(6):558-66.
PMID: 18511942
In this study, the researchers found that EGCG efficiently inhibits the formation of both alpha synuclein and Alzheimer’s associated beta amyloid fibrils. And it did this by directly binding to the native unfolded forms of the protein and prevented their conversion into the toxic forms of aggregates.
And other studies have reported even more impressive results:

Title: EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity
Authors: Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE.
Journal: Proc Natl Acad Sci U S A. 2010 Apr 27;107(17):7710-5. doi: 10.1073/pnas.0910723107.
PMID: 20385841 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers found that EGCG has the ability to not only block the formation of of alpha synuclein fibrils and stabilize monomers of alpha synuclein, but it can also bind to alpha synuclein fibrils and restructure them into the safe/non-toxic forms of aggregates.
And more recently, other researchers have extended these results:
Title: EGCG-mediated Protection of the Membrane Disruption and Cytotoxicity Caused by the ‘Active Oligomer’ of α-Synuclein.
Authors: Yang JE, Rhoo KY, Lee S, Lee JT, Park JH, Bhak G, Paik SR.
Journal: Sci Rep. 2017 Dec 20;7(1):17945.
PMID: 29263416 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers reported that EGCG facilitates the formation of a non-toxic oligomer form of alpha synuclein, and protects cells from the membrane disrupting toxic form of aggregated alpha synuclein.
And just when it sounds like EGCG is the perfect compound for Parkinson’s, another very recent study reports that EGCG also modulates the response of peripheral immune cells in a model of Parkinson’s:
Title: (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease.
Authors: Zhou T, Zhu M, Liang Z.
Journal: Mol Med Rep. 2018 Apr;17(4):4883-4888.
PMID: 29363729 (This report is OPEN ACCESS if you would like to read it)
In this study the researchers used a neurotoxic mouse model of Parkinson’s (MPTP) to assess the neuroprotective potential of EGCG. They found that EGCG not only protected dopamine producing cells (the cells most affected by Parkinson’s), but also reduced the levels of inflammatory factors (such as tumor necrosis factor-α and interleukin-6) in the peripheral blood of the mice.
There is a lot of evidence accumulating that EGCG is a really interesting compound for Parkinson’s (and that is the third use of the word ‘interesting’ in this post if you are keeping count, Chris).
Has EGCG been tested in the clinic for Parkinson’s?
Yes, it has.
The efficacy and safety of EGCG was tested in 480 de novo (recently diagnosed) people with Parkinson’s in China in a study supported by the Michael J Fox foundation (Click here to learn more about the details of that trial). The study was started in 2007, and the participants were to be treated for 1 year with one of three different doses of EGCG or a placebo treatment.
The results of this trial have not been published, but the Michael J Fox Foundation have a short summary on their website which reads:
To evaluate the safety, tolerability and efficacy of green tea polyphenols (GTPs) in slowing disease progression in patients with early PD, the team carried out a multi-center, double-blind, randomized, placebo-controlled, delayed-start study in 32 Chinese Parkinson Study Group (CPSG) sites. Study enrollees were 410 untreated PD patients with disease duration of less than five years and Hoehn & Yahr stage below 3 who were not heavy tea drinkers. Participants were randomized to one of three doses of GTP (0.4, 0.8 or 1.2 grams daily given in two equal oral doses) or matching placebo. After six months, placebo was switched to 1.2 grams daily of GTP. All patients were treated for 12 months.
The change in total UPDRS score from randomization to six months was significantly improved in GTP-treated groups as compared to the placebo group, but this was not observed at 12 months. In the delayed-start group, the change in UPDRS from six months (the start of active GTP) to 12 months was significantly improved. Insomnia was slightly increased in GTP-treated patients, but there were no other significant differences in adverse effects. GTP is well tolerated and appears to provide, at least, a mild symptomatic benefit in early untreated PD. (Source)
There is also a clinical trial just being completed, which is testing EGCG in multiple system atrophy (or MSA a condition very similar to Parkinson’s – Click here to read a previous SoPD post on MSA).
This more recent trial is called the PROMESA study:
Title: The PROMESA-protocol: progression rate of multiple system atrophy under EGCG supplementation as anti-aggregation-approach.
Authors: Levin J, Maaß S, Schuberth M, Respondek G, Paul F, Mansmann U, Oertel WH, Lorenzl S, Krismer F, Seppi K, Poewe W, Wenning G; PROMESA study group, Giese A, Bötzel K, Höglinger G.
Journal: J Neural Transm (Vienna). 2016 Apr;123(4):439-45.
PMID: 26809243
In this study, 92 individuals with MSA have been randomly assigned to either the treatment group (EGCG) or the control group (Placebo – click here to read more about the details of the study). The study has evaluated the safety, tolerability and a potential disease-modifying effect of EGCG in the participants. We are currently awaiting the publication of those results (expect a follow up post when those results get published).
And as far as I’m aware, these are the only clinical studies of EGCG in the context of Parkinson’s (and again, I’m happy to be corrected here).
Why aren’t there more clinical studies of this compound?
Quite simply: It’s not patented.
There’s no protective advantage for any biotech firm/pharmaceutical company to try and take this compound through the expensive clinical trial process.
But numerous clinical studies for various medical conditions have suggested that EGCG is well tolerated. Oral administration of EGCG is rapidly absorbed through the gut (Source), and it can cross the protective blood–brain barrier (Source), so it is a shame that more research is not being done with this compound.
What is the best source of EGCG?
There are solid supplements of EGCG available, but these have recently been called into question.
The European Food Safety Authority (EFSA – the agency of the European Union that provides independent scientific advice and communicates on existing and emerging risks associated with the food chain) has recently warned that food supplement doses of EGCG at 800 mg or more per day may be associated with signs of liver damage.
They are quick to reassure that this is only related to food supplements. Liquid infusions of EGCG appear to be safe (Click here to read more about this).
Liquid infusions of EGCG?
So Green tea is the best ‘liquid infusion’ source of EGCG.
Green tea. Source: BBC
But there is a great deal of variability in the amounts of EGCG across the different brands of green tea.
Consumerlab (which provides independent test results, reviews, ratings and comparisons of vitamins, supplements, herb and nutrition products) recently conducted an analysis of EGCG levels in different brands of green tea, and their results suggested that Teavana‘s Gyokuro green tea contains 250mg of catechins (1/3 of which is EGCG), as well as high levels of caffeine.
Remarkably, even some plain, old supermarket brands of green tea (such as ‘Lipton green tea bags’) also contain good levels of EGCG.
But be warned! According to the report, a lot of green tea products contain little – if any – EGCG, such as Diet Snapple Green tea. So caveat emptor.
What is the best way to prepare green tea to maximise the EGCG content?
To maximize EGCG content i your cuppa, one is advised to pour boiling water (not just hot water) over green tea leaves or tea bag, and let it sit for at least 10 minutes.
Are there other natural sources of EGCG?
Source: Sciencemag
Strawberries, raspberries, blackberries, plums, peaches, kiwi, and avocado all contain small amounts of EGCG, but green tea appears to be the best source.
So what does it all mean?
Quite simply, it means that I’m off to brew a pot of de-caffeinated green tea (most of the few side effects associated with green tea relate to caffeine), while we wait for the results of the PROMESA clinical study.
Cheers!
EDITORIAL NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. Dietary changes can impact the effectiveness of treatments. PLEASE speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post is sourced from dailymatcha












Wow – great post, Simon! I’m definitely adding green tea to my afternoons! What about chocolate, red wine, and fruit – do they show the same levels of EGCG? I already eat blueberries, walnuts,seeds (pumpkin and sunflower), cinnamon, turmeric, blk pepper with yogurt in the morning – would be happy to add a glass of red wine every night (now have it once or twice a week)! Not to mention a nice piece of dark chocolate before bed…
Serious question – what if supplements such as TUDCA and EGCG are the answer to pausing/stopping Parkinson’s but because they cannot be monetized by Big Pharma, no one will do clinical trials on them, and without clinical trials doctors ignore and are skeptical of supplements, despite research and anecdotal evidence? If supplements which are readily availalbe are the key, and not drugs with horrible side effects, how do patients get access to that information if doctors are reluctant to proscribe?
LikeLike
Hi Nadra,
Thanks for your comment – glad you liked the post.
There has actually been research into the benefits of chocolate for Parkinson’s (https://scienceofparkinsons.com/2016/12/27/mmmm-chocolate/), but the blood pressure lowering aspect of it is an issue for PD, which is characterised by low blood pressure. If you are interested in potential dietary interventions, you could have a look at the research of Dr Laurie Mischley (https://scienceofparkinsons.com/2017/09/18/food/) – she is very nice and happy to talk nutrition.
As for clinical trials, there have been and are ongoing efforts for these compounds, but most of them are academic research groups conducting the studies which limits their scale. Until a more effective version of the Clinicrowd initiative(https://scienceofparkinsons.com/2018/05/30/mannitol/ – #shamelessselfpromotionhere) becomes available, it will be slow going getting any of these unpatented supplements through the clinical trial process. Opportunity for someone.
Kind regards,
Simon
LikeLike
Is Cliniccrowd only interested in testing Mannitol, or will it also be looking at other supplements or supplement mixes? As I mentioned in a reply to a previous post, I have been taking a supplement mix for 8 months that includes TUDCA and EGCG, and during that time some symptoms have regressed and there has been no progression. It frustrates me that as you said in a different post, “There are a great number of compounds that could be potentially interesting for Parkinson’s and yet they sit on the sidelines, because there is no economic incentive for pharmaceutical companies to take them to the clinic for testing.” Therefore doctors do not tell patients about them because while there have been academic studies on them they have not been put through the clinical trial process. These supplements could be helping people right now – and for the most part they have very minor side effects – especially compared to drugs.
LikeLike
Hi Nadra,
Thanks for your comment. It is a very frustrating situation, but there are companies trying to change this – for example, Amylyx Pharmaceutical Corp (http://amylyx.com/) which is developing AMX0035 (a combination of Phenylbutyrate and TUDCA). Clinicrowd are not just focused on mannitol, that was simply their pilot project. I am sure that they would be interested in discussing other projects. Critically though, the PD community requires better methods of self assessment, which can help to determine if something is having a beneficial effect or not (rather than simply trying to decide in one’s head if I feel better). I think there is lots of opportunity for the PD community to make a major difference here.
Kind regards,
Simon
LikeLike
Simon,
Thank you. Once again you’ve come up with something very interesting for us PwP to think about.
Before switching on my kettle I would like, as a sanity check, to see PD epidemiological data for:
– non-tea drinkers v tea drinkers;
– areas where black tea is the norm v areas where green tea is the norm.
John
LikeLike
Hi John,
Thanks for your comment – glad you liked the post.
So I didn’t touch on the epidem. research because that kind of analysis is complicated in this setting. There are different levels of catechins, etc in each cup of tea, let alone between brands (not to mention different levels of caffeine in each tea confusing things further – caffeine being a PD risk reducer). And this shows itself in a lot of the data on this topic that has been published – for example, there was a study in which Green tea drinking was found to be unrelated to Parkinson’s risk (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2737529/)
A large meta-analysis did show that tea drinking can lower the risk of PD, but no apparent dose-response relationship was found (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3289976/). And this has been reported in other studies as well (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3289976/ & https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3320056/ & https://www.ncbi.nlm.nih.gov/pubmed/21458354).
There is a lack of data (as far as I’m aware) that focuses specifically on green tea and risk of PD (or benefits for people with PD), and I would be happy to be corrected on this. I have often thought that there is probably an opportunity for someone in the western world to conduct a study on this. Given that green tea is not a staple part of most western diets, it would be a relatively clean intervention, and it could even be conducted by the community (perhaps a Clinicrowd/mannitol-type effort – just thinking out loud here).
I’m not sure if this fulfils the definition of sanity check, but hopefully it helps.
Kind regards,
Simon
LikeLiked by 1 person
Great work you are doing here Simon on Parkinsons
LikeLike
Thanks Kelvin – Glad you like it
LikeLike
Simon,
Seriously love your site. Has very much helped my wife and I manage her early onset parkinsons with clarity and hope for the future. A little information (well, in the case of The Science of Parkinsons, a LOT of information) can go a long ways. That is also why I have brought it forward to our neurologist and our local PD support group as proposed “required reading”. Anyways, I was just wondering what you would think about all the green tea supplements that are out there. Things like extracts, etc. I know excess is best avoided in almost all situations, but would there be any benefits in modest supplements or is a cup of green tea a day the safest, most efficacious approach? Thanks.
LikeLike
Hi Gavin,
Thanks for your kind words regarding the website – sorry to hear of your wife’s diagnosis, but well done for taking a proactive approach and trying to learn more about PD. I I hope the medication is helping her.
Regarding green tea supplements, as we said in the post, the European Food Safety Authority (EFSA) is currently investigating the solid supplements so we’ll wait to see what they report before making any comment. Sorry, this isn’t much of an answer to your question, but at present we simply do not know the answer.
Kind regards,
Simon
LikeLike
Green tea matcha lattes used to be a favorite until I realized the calcium was probably counter productive. Maybe soy matcha lattes?
LikeLike
Sorry Don, but soy milk generally has as much calcium as dairy milk: http://www.soyfoods.org/myth/soymilk-protein
Back to the drawing board?
LikeLike
There is an excellent 2008 review article titled “Simultaneous Manipulation of Multiple Brain Targets by GreenTea Catechins: A Potential Neuroprotective Strategyfor Alzheimer and Parkinson Diseases” that can be found here:
https://mafiadoc.com/simultaneous-manipulation-of-multiple-brain-targets-by-green-tea-_59d0a3631723ddd586192054.html
According to this article, the effects of EGCg on inhibiting alpha-synuclein aggregation are due to its ability to chelate iron from the brain, so perhaps that is the specific means through which it performs that valuable service. We know that iron has been found in abnormally high quantities in the substantia nigra areas of PD patients.
Here are some pertinent excerpts from that article:
—
In spite of the lack of systematic clinical trials with tea polyphenols in neurodegenerative diseases, human epidemiological and new animal data suggest that tea consumption inversely correlates with incidence of dementia, Alzheimer disease (AD), and Parkinson disease (PD).
In elderly Japanese subjects, it was found that higher consumption of green tea is associated with a lower prevalence of cognitive impairment, and in the United States, people that consumed 2 cups/day or more of tea presented a decreased risk of PD…
In consensus, a recent prospective 13-year study of nearly 30,000 Finnish adults demonstrated that drinking three or more cups of tea is associated with a reduced risk of PD…
[T]he antioxidant/metal-chelating attributes of catechin polyphenols alone are unlikely to be an adequate explanation for their neuroprotective and neurorescue capacity…
Neurodegeneration in PD and AD or other neurodegenerative diseases… appears to be multifactorial, whereby several mechanisms are implicated in a cascade of events involving many biochemical and signaling pathways. Common features involve impairment of protein handling and aggregation associated with dysfunction of the ubiquitin–proteasome system (UPS), depletion of endogenous antioxidants, reduced expression of trophic factors, inflammation, glutamatergic excitotoxicity, and induced expression of proapoptotic proteins and increase of iron and nitric oxide levels, leading to oxidative stress (OS) damage…
Iron content alteration has been described in brains of PD and AD patients, which may be caused, to a large degree, by endogenous dysregulation of iron uptake, transport, distribution, and storage. Iron is one of the most essential transition metals involved in the formation of oxygen-free radicals…
Accumulation of iron, specifically in the SN pars compacta (SNpc) is one cardinal feature of PD and is considered to be a major contributor to OS [Oxidative Stress]….
Considering the diverse etiological nature of AD and PD, drugs directed against single functional components of the different disease pathologies, such as cognition or movement disorder, will be limited in efficacy. A novel therapeutic approach gaining large acceptance focuses on the implementation of a cocktail of drugs, or a single molecule, possessing two or more active neuroprotective moieties that simultaneously target different disease mechanisms. Accumulating new data suggests that green tea catechins may well fulfill the requirement for a putative neuroprotective drug because of their diverse pharmacological activities…
[A] green tea polyphenol extract or individual EGCG prevented striatal DA depletion and SNpc dopaminergic neurons loss when given chronically to mice treated with the parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)…
These findings receive further support from our recent animal studies, where EGCG was shown to restore nigrostriatal DA neuron degeneration when administered to mice post-MPTP (unpublished results)…
Tea catechins are phenolic compounds, and as such, they possess the ability to chelate transition metal ions, thereby preventing the formation of iron-induced free radicals and acting as powerful hydrogen-donating radical scavengers of reactive oxygen and nitrogen species (ROS and RNS, respectively)…
The neuroprotective effect of green tea polyphenols in vivo may also involve the regulation of antioxidant protective enzymes. EGCG was found to elevate the activity of two major oxygen radical species-metabolizing enzymes, SOD and catalase in mice striatum…
Our pioneer in vitro cell signaling studies revealed a specific involvement of the PKC pathway in the neuroprotective mechanistic action of EGCG. PKC has a fundamental role in the regulation of cell survival, programmed cell death, long-term potentiation (LTP), and consolidation of different types of memory…
The observations that iron induces aggregation of inert α-synuclein and Aβ peptides to toxic aggregates have reinforced the critical role of iron in OS-induced pathogenesis of neurodegeneration, supporting the notion that a combination of iron chelation and antioxidant therapy may be one significant approach to neuroprotection…
Similar to Aβ, α-synuclein associated with presynaptic membranes is not toxic, while in the presence of iron, it forms toxic aggregates that are considered to contribute to the formation of LBs via OS. Thus, the radical scavenging and free iron-complexing activities of green tea polyphenols may directly influence aggregation and deposition of either Aβ or α-synuclein in brains of AD and PD patients, respectively. Indeed, it was shown that several metal-binding natural antioxidants, including polyphenols of green tea and wine (e.g., resveratrol, myricetin, (+)-catechin, (-)-epicatechin) inhibit formation of nascent Aβ and α-synuclein fibrils and elongation of the fibrils, and promote destabilization of the formed assemblies…
Earlier viewed as simple radical scavengers, green tea catechin polyphenols are at present considered as multimodal acting molecules invoking a myriad of cellular neuroprotection/neurorescue mechanisms involving iron chelation, scavenging of oxygen and nitrogen radical species, and activation of PKC signaling pathway and prosurvival genes. Their nontoxic, lipophilic (and thus, brain permeable) nature is advocated for “ironing out iron” from those brain areas where it preferentially accumulates in neurodegenerative diseases.
—
LikeLike
Hi Lou,
Thanks for the interesting comment. Hopefully we’re not breaking too many copy write rules here. Very interesting though.
Kind regards,
Simon
LikeLike
That was just the link I had in my notes, but Semantic Scholar
https://allenai.org/semantic-scholar/
https://www.geekwire.com/2017/ai2-semantic-scholar-biomedicine/
(a project of the Allen Institute) has a better (i.e., searchable) PDF for the same document, and I doubt that they would be hosting pirated papers. Please feel free to edit my comment to substitute their link, which is more legible and also searchable inside the document:
Click to access 253a02fa7fea9d082393af22db179f01ed02.pdf
LikeLike
As a caregiver for a PD patient, I think your decision to concentrate on what the study says about the two positive control substances, EGCg and baicalein, serves patients’ interests well, as the esoteric compounds found to be *most* suppressive of alpha-synuclein aggregation are probably not available for therapeutic use in patients, and may not even be safe for such use.
I would love to know by what means these researchers were able to test so many compounds – clearly not by anything requiring manual activity per substance! But, staying with the more pertinent focus that you’ve identified, I moused around and found some of the charts from the study that Google had somehow extracted from behind the study’s paywall, to see how EGCg and baicalin rate among the substances tested:
https://www.google.com/search?q=%22We+screened+746,000+compounds,+leading+to+58+hits+that+markedly+inhibit%22&source=lnms&tbm=isch&sa=X&ved=0ahUKEwjF8uvAr8TdAhVmxlQKHc4RAwsQ_AUIDigB&biw=1884&bih=896#imgrc=dtNgaUSyJXF2eM:
If I’m reading this correctly, then the Y axis in these charts must be some kind of measure of aggregation, since Tht fluorescence can be used to measure protein aggregation:
https://en.wikipedia.org/wiki/Thioflavin
“When it binds to beta sheet-rich structures, such as those in amyloid aggregates, the dye displays enhanced fluorescence…”
If that’s correct, then one interesting fact revealed by this chart is that while EGCg does a great job of suppressing aggregation, baicalein does that even better. It would be interesting to see how well both of them do it together, if anyone has bothered to test that combination.
My partner has been using both substances for some time for her PD. Actually, instead of baicalein, she’s using baicalin, which is what ingested baicalein gets converted to within the body after ingestion, because we could not find baicalein for sale anywhere, except on sites aimed at researchers, who presumably are injecting it into happy little rodents.
Both baicalein and baicalin are flavones (components) of the Chinese herb Scutellaria Baicalensis, but ever since Ron Teeguarden herbs changed its Scute formulation to basically pure baicalin, I haven’t been able to find a good source for the extracted whole plant. However, since baicalin/baicalein are the most studied and probably most important of the plant’s flavones, we have been very happy with the (much more economical) baicalin powder sold by a company called Liftmode. They send out a certificate of analysis with each order, and the rich yellow color of the supplement just has a good feel to it (that’s very subjective, but it just seems like a quality product). It doesn’t taste bad, and we stir it into juice laced with various supplement powders each morning and also each evening.
So if PD patients would like to try some baicalein for its various properties, and can’t find it as a supplement for sale, if I’m not mistaken (and please do correct me it this is wrong) baicalin, which is much more readily available to consumers (as opposed to researchers) probably delivers more or less the same benefits.
For EGCg, my partner is taking 200mg, one in the morning and one in the evening, of EGCg made from decaffeinated green tea, sold by Now foods. So even if the concerns about liver damage at 800mg daily turn out to be correct, hopefully that dose is low enough not to encounter such hepatotoxicity.
Since the premise of toxicity seems to be based on particular cases of liver damage, I have always wondered whether the particular supplements causing such contamination might have been contaminated with something that caused the damage. I have never been able to find a list of the actual products that were responsible for the instances of liver damage reported, and would be interested to know if anyone has ever tested the premise more rigorously then by just looking at these particular incidents (e.g., by using actual EGCg from a known pure source).
But anyway, 400mg per day, even according to this report, should be OK, I suppose. She takes EGCg in that amount every day, and has done so for several years. Unfortunately, the Now EGCg product we use is not made from organic green tea, and I haven’t been able to find an organically-sourced EGCg product. I wrote to Now and they replied that “the green tea is not certified organic. We test for pesticides, heavy metals, microbes and other contaminants, though. This is a water and alcohol extraction process.” Now is a well-known company and I use a number of their products, so I guess I have to go with their reputation on this.
Nonetheless, I would love to find an organically-sourced EGCg that is also from decaffeinated tea, if anyone knows of one.
LikeLike
Ah Lou, you are stealing my thunder.
A post on baicalein is currently in the works – maybe it will be ready next week. I did not want to do both EGCG and baicalein in the same post as these posts are already ridiculously long. EGCG was simply more accessible for most readers so we began with that.
Thanks for sharing the information regarding your experience with EGCG etc. I would be interested to know how you are assessing your partners condition while taking some of these supplements etc. How are you measuring a response? And this question is not academic, I am genuinely interested. One of the most frustrating communications I get from readers is ‘Hi Simon, I have started taking Nico Ribo, and I think I feel better’. My immediate response is polite, but I am always inclined to ask ‘based on what?’ What measure says you have had a response from the compound? If they wrote that they have started taking supplement X and they noticed that their REM pattern or their blood pressure has improved, that would be extremely useful information. Thus, I am always curious to know what methods of assessment folks have devised.
Any thoughts on this would be greatly appreciated. And I will try to find some time to respond to your previous comments – things are just a bit la vida loca at the moment.
Kind regards,
Simon
LikeLike
I think your readership for that article will be about 10,000 times that of my little comment. And will contain much more complete information.
I’m so glad you asked about how we can know that the various supplements we are taking to slow progression are actually having their intended effect. I’ve thought quite a bit about that, and it’s a difficult question to answer, but also a very important one to address. And there are a number of difficulties in addressing it.
First of all, if your goal is to halt progression (rather than to ameliorate symptoms), then there may be absolutely no apparent effects initially from ingesting a given set of supplements, even if they are having their intended efffect and have *completely* halted all further progression. There *may* be some immediate improvements noticed, if, say, some of the patient’s dopamine neurons that were compromised but not yet dead, start to function more capably, but that’s not the main goal, and any such effects are likely to be minor, and would occur as only a single increment close to the start of the therapy which would not be repeated. Because by the time of a PD diagnosis, the majority of those neurons have already died, and a therapy that is intended to halt progression is not going to bring those neurons back from the dead.
Therefore, it’s only after significant time has elapsed that one could notice some difference between what one might *expect* to occur *without* the treatments, and what has *actually* occurred.
But even then, you have to ask “as compared to what?” You are in effect running a study where n=1, with no control group at all.
And even if you have zero progression over the course of several years, how do you know that the responsible factor isn’t something else that you happened to be doing at the same time?
The most you will ever have from such an experiment is a single anecdotal report.
So, then, the goal of such therapy is not so much to gather evidence of efficacy, as to have a beneficial effect for the patient under treatment. Because the disease *is* progressing, there *is* no known cure, and *something* has to be done if you want to avoid the otherwise inevitable decline under the present standard of care, which is almost entirely palliative.
So what I *am* doing is crafting a hypothesis, consisting of a model of how the disease process is maintained, and then crafting a set of therapies that are collectively intended to disrupt that disease process. The hypothesis will be based upon available research, and the predicted effects of the individual therapies, which allow them to play their assigned roles in the intervention, will be based upon available research, but the combined effects of those therapies upon the model will be based upon conjecture.
Most existing research seems aimed at isolating and evaluating the effects of a single therapy, rather than a combination of therapies based on a particular disease model (which is my own approach to treatment). Perhaps this model for studies has been influenced by the desire to develop evidence for the efficacy of specific drug products that are to be presented to the public for sale. Or maybe it is because the combinatorics of testing multiple approaches at once tend to get out of hand – although I think that might be addressed by pursuing a strong hypothesis about the disease process.
For whatever reasons, most studies seem to be of monotherapies. And yet, as mentioned in the quote from the Green Tea Catechins article at the end of one of my other comments here, it is likely that any successful treatment for PD will involve multiple substances, with multiple mechanisms of action, which intervene in the disease process in a variety of ways simultaneously.
The therapy that my partner is using, for example, involves not just EGCg and baicalin, but also ubiquinol, NAC, Longvida curcumin, acetyl-l-carnitine and alpha-lipoic acid. A complete account of the supplements we are using to deter progression (along with the model on which treatment is based, and with links to papers intended to support the efficacy of the various supplements) can be found on the Smart Patients site here:
https://www.smartpatients.com/conversations/41861
I think viewing it may require signing up for the site, which is free. I recommend the site – it has an excellent search function – searching for “Science of Parkinson’s”, for example, brings up a number of patient discussions of articles on this site.
So, again, you have to go out on a limb and make some assumptions regarding what is likely to work. You try to minimize risk by looking at safety studies for each of the substances you intend to employ, and looking for any interactions that might occur between pairs of substances. And then you invest in an approach that is based on your best available model, and *hope* that ten years down the road your patient is still walking around with less impairment than might otherwise be the case. So, you are doing all of this in the hope of never finding out exactly how bad things might otherwise have been – and that also means never finding out for certain how *good* the therapies that you decided upon actually were.
Now, my partner is presently 12 years out from her diagnosis, and she has definitely gotten worse in the course of those 12 years. But we are still walking around, eating out, going to the gym, and generally living a more constrained but still somewhat normal life together.
In the past year, she has started to show signs of dysautonomia. The most troubling of these signs is neurogenic orthostatic hypotension (nOH), which was starting to make her feel faint at times.
I see dysautonomia as related to the spread of the disease breadth-wise through brain areas that are outside the substantia nigra area. As mentioned in another comment on this page, I’ve only recently been paying closer attention to this process, having concentrated most of my efforts on addressing the neuroinflammation within the substantia nigra area. We got lucky inasmuch as the baicalin, EGCg and curcumin, which are excellent anti-inflammatories within the SN area, also happen to be good preventers of alpha-synuclein aggregation.
Still, apparently, they are not enough to stop the advance of the illness throughout the brain, or my partner would not be experiencing these autonomic nervous system symptoms. It seems possible that if I had paid more attention to this process earlier in her illness, we would have found other things to address it, and she would be in a better place than she is now. And I have still not developed a good approach to slowing the neuron-to-neuron spread of the disease, beyond the three substances just mentioned.
Still, we have managed to address the autonomic symptoms that have appeared thus far. In this we were aided by the fact that I had already been through nOH with my father, who had that problem simply because of his advanced age, and so I had already discovered a good medication, called vinpocetine, which is a phosphodiesterase (PDE) inhibitor that works on the kind of PDE (I think there are five kinds) that constricts the part of the circulatory system that takes blood to the head. It’s over the counter in the U.S., but in the U.K. it is prescription-only (because it’s very serious medicine, not to be taken on a whim), and is sold there under the name Cavinton.
Anyway, it worked very well in her case, and she has very little difficulty now with the nOH. Although I would note to anyone reading this that it affects blood viscosity and clotting, and thus can alter the effects of anticoagulant therapy, and should definitely be checked with a physician before trying it in any case, even if it is available over-the-counter where you are located.
That leaves only some occasional excessive perspiration, which we’ve largely remedied by running air conditioning (a remedy that’s kind of an end-run around the dysautonomia, but it works). Tremor is still well-controlled when on medication. If she’s late to take a dose of levodopa, though, the tremor will appear quite reliably. No dyskinesia at all, really. So, I think we’re doing pretty well, given the nature of the illness, which is to say: not well at all.
But this is very subjective, and so about nine months ago (at 11.5 years from diagnosis), I decided to try to get some idea of how she is doing as compared to the “average PD patient,” and then to use that to project where she might be in future years if she continued to be at the same percentile relative to other patients as the years unfold. In some ways, a comparison of her progression to what is considered “average” might then provide a (rather poor) substitute for a comparison to a control group in a more formal study. So you see, I am still trying to answer your question, even while saying that it is not possible to do so.
(This is where my calculations get very flaky and back-of-the-envelope, but I don’t think they’re entirely off-base…)
Looking around, I found only one study of PD progression addressing the time to reach each stage, from 2006, called Prognosis of Parkinson’s Disease: Time to Stage III, IV, V, and to Motor Fluctuations. This study can be found here:
Click to access 1a0a73bfc04787db873d0d08b1e2eb699e57.pdf
This study involved 1768 PD patients, and used the older Hoehn and Yahr scale to measure disease progression. I decided to use the more recent and accurate UPDRS to evaluate her condition, and then to map that UPDRS value to a Hoehn and Yahr stage. According to my reading of this article:
https://onlinelibrary.wiley.com/doi/full/10.1002/mdc3.12476
the mapping of UPDRS to Hoehn and Yahr stages is roughly as follows:
MIDDLE of HY Stage 1 equals UPDRS 3.8
MIDDLE of HY Stage 2 adds 7.7 and thus equals UPDRS 11.5
MIDDLE of HY Stage 3 adds 14.6 and thus equals UPDRS 26.1
MIDDLE of HY Stage 4 adds 2.0 and thus equals UPDRS 28.1
BEGINNING of HY Stage 5: study does not provide this, but interpolation suggests that this stage would START around 29.1
I measured my partner’s UPDRS using the following online calculator/questionnaire:
http://farmacologiaclinica.info/scales/UPDRS-PARKINSON/
At that time, her UPDRS was 15.
So, interpolating, we find that she is 24 percent of the way from the middle of stage 2 to the middle of stage 3.
So, her Hoehn and Yahr stage, then, would be about 2.74.
Figure 2 of the above study shows that at 11-15 years from diagnosis, 2.4 percent were at stage 1 or less (i.e., <= the start of stage 2), and 28.7 percent were at stage 2 or less (i.e., <= the start of stage 3).
That same figure shows that at 6-10 years from diagnosis, 7.1 percent were at stage 1 or less (i.e., <= the start of stage 2), and 41.9 percent were at stage 2 or less (i.e., <= the start of stage 3).
Interpolating, we could say that the 11-15 year numbers pertain to a person who is on average 13 years from diagnosis, and the 6-10 year numbers pertain to a person who is on average 8 years from diagnosis. Therefore, an average person 11.5 years from diagnosis would be assumed to be about 70 percent of the way between the 6-10 year numbers and the 11-15 year numbers.
From this interpolation we get that for persons at 11.5 years from diagnosis: 5.69 percent were at stage 1 or less (i.e., <= the start of stage 2), and 37.94 percent were at stage 2 or less (i.e., <= the start of stage 3).
Since my partner is at 2.74, interpolating yet again, 74 percent of the distance between 5.69 and 37.94 is 29.555.
So, this *very* crude reasoning, with its multiple interpolations, suggests that about 70.445 percent of patients at 11.5 years from diagnosis were doing worse than my partner, and about 29.555 percent were doing better.
I've forgotten most of my college statistical training, and thus have relied heavily on crude interpolations, and I may have even made some mistakes on those (for example, in dealing with the granularity of open and closed intervals as they pertain to intervals spanning years from diagnosis). But that was the best that I could do; any corrections you might provide would be most welcome.
I do think that the above gives *some* idea of how a given patient's condition compares to where patients generally are at a certain number of years from diagnosis. And from this you might even be able to perform a crude projection of where those same patients are likely to be some year into the future.
For example, we see from the above that at 13 years from diagnosis (the middle of the 11-15 year range) 28.7 percent were still below stage 3. Since my partner is in the top 30th percentile, we might infer that at 13 years (in about another nine months) she will have just entered Hoehn and Yahr stage 3.
For reference, the Hoehn and Yahr stages are described as:
Stage 0: No signs of disease
Stage 1.0: Symptoms are very mild; unilateral involvement only
Stage 1.5: Unilateral and axial involvement
Stage 2: Bilateral involvement without impairment of balance
Stage 2.5: Mild bilateral disease with recovery on pull test
You can see that my partner is in fact at this stage
Stage 3: Mild to moderate bilateral disease; some postural instability; physically independent
Stage 4: Severe disability; still able to walk or stand unassisted
Stage 5: Wheelchair bound or bedridden unless aided
Such a prediction of future symptoms, however imprecise, could be useful for planning purposes. For example, we have some stairs in our living situation. So, since the big difference in Stage 3 is postural instability, I'm thinking that maybe we should be looking to change our living situation to eliminate the stairs by around the time she would reach stage 3.
OTOH, some of the supplements we are taking we were not taking until the last several years. So we may have improved our treatments for the illness, and that *could* mean that the disease is progressing more slowly now than it was in her earlier years, throwing off the above estimates. Certainly, she is not showing any signs of postural instability at the present time.
So, we hope for the best and continue to examine new treatments. I have obtained some TUDCA based on reading your article and a number of anecdotal reports, but my partner already has so many pills to ingest (even after putting as many as possible in powder form) that adding that therapy has been delayed for the moment.
LikeLike
I initially became aware of EGCg and baicalin/baicalein while attempting to disrupt the innate immunity feedback loop that, according to my own understanding of PD, is responsible for much of the damage to dopamine neurons in the substantia nigra. Our specific target was in the neuroimmune system, as opposed to the peripheral immune system that was the subject of the new and fascinating Molecular Medicine Reports study to which you have linked in the article above. In contrast to that study, we were trying to influence stuff that is all happening on the brain side of the blood-brain barrier.
So my partner and I started out using these substances primarily for their anti-neuroinflammatory (rather than anti-aggregation) effects, in the hope that they will interfere with the autoimmune feedback loop that goes from damaged neurons, to the alpha synuclein and neuromelanin those neurons dump out into the inter-cellular space as they die, to the receptors on nearby microglia (part of the innate immune system) that are triggered into their “Mr. Hyde” classical M1 attack type of activation by those substances, to the inflammatory cytokines (e.g., TNF-alpha) produced by those activated microglia, back to other, formerly-healthy “bystander” neurons that get damaged by the cytokines, which starts the cycle over again.
Microglia have a number of receptors on their exteriors that sense the presence of substances deemed to be evidence of invasive organisms. I have read that alpha synuclein activates microglia in part through the latter’s TLR2 receptors, causing the microglia to deem the alpha synuclein to be a threat, and launch an attack against it, which damages nearby neurons.
And while alpha-synuclein has a greater effect on those receptors when it is aggregated, even non-aggregated alpha-synuclein can have that effect to a significant degree, when it has been dumped into the inter-cellular space as a result of damaged neurons spilling their contents into the surrounding environment. So aggregation, while a factor, is not of primary importance to this particular type of inflammation.
Neuromelanin can *also* activate the TLR2 microglial receptor when it is dumped out of damaged neurons. And alpha-synuclein also can activate *another* important receptor, TLR4.
Both EGCg and baicalin/baicalein have the ability to inhibit TLR2, and baicalin can also inhibit TLR4. Thus they can, in theory, interfere with the autoimmune feedback loop that goes through innate immunity, by reducing the degree of microglial activation. And they can also interfere, through their antioxidant action, with the cascade of neuronal destruction that is set off by the cytokines (like TNF-alpha) that are produced by the activated microglia.
So this would seem to provide a separate mechanism for protecting against the ravages of PD, in addition to the anti-aggregation effects that have been described in this article, which seem to confer their benefits most importantly within neurons, as opposed to the inter-cellular space, where aggregation is apparently only a secondary factor in the neuroinflammation occurring through the feedback loop of innate immunity.
So, apparently there are two aspects of the disease process at work – one within neurons, and one in the extra-cellular space, and these two therapeutic substances seem to play a positive role in interfering with both of those aspects of PD’s pathogenesis. And so, they would seem to slow PD progression in what may be two somewhat distinct, if related, ways.
I’ve been trying to piece together a picture of PD progression, and it’s very hazy at present, so I hope to get some feedback regarding how accurate the following might be. But according to my present understanding, the concerns covered in this article about alpha-synuclein aggregation and Lewy Body formation, relating to processes that happen mainly inside individual neurons (formation of Lewy bodies from aggregated alpha-synuclein) have to do with the spread of PD from one neuron to nearby neurons (perhaps via nanotubes connecting infected cells to other cells, as your report from last year indicates):
So, this is part of the slow spread of PD through the various parts of the brain, as Braak was attempting to describe in his hypothesis.
When this gradual expansion reaches the substantia nigra area, it encounters conditions much more conducive to autoimmune inflammation (in part due to the presence of neuromelanin there), and so a self-sustaining inflammatory “fire” of sorts breaks out in the *extra*-cellular space within that area, involving activated microglia, as described above. And it is only once that fire consumes perhaps 70 percent of the dopamine neurons in that area that most patients get the motor symptoms that result in a diagnosis of PD.
So it seems that there are two related disease processes, one of which occurs inside neurons (and spreads through direct conduits between them), spreading slowly through a fairly large expanse of the brain (and causing a number of non-motor effects, such as loss of smell in the olfactory bulb, and acetylcholine loss in the nucleus basilis area) and the other of which is a more intense inflammatory process that is highly localized to the inter-cellular space of the substantia nigra area.
The marvel of EGCg and baicalin, is that they *each* seem to have benefits for *both* of these kinds of disease perpetuation and propagation (plus, as the new study you supplied indicates, peripheral immunity effects as well). That review article I linked in an earlier comment sums it up very nicely as follows for EGCg (and the same could be said of baicalin):
“Considering the diverse etiological nature of AD and PD, drugs directed against single functional components of the different disease pathologies…will be limited in efficacy. A novel therapeutic approach gaining large acceptance focuses on the implementation of a cocktail of drugs, or a single molecule, possessing two or more active neuroprotective moieties that simultaneously target different disease mechanisms. Accumulating new data suggests that green tea catechins may well fulfill the requirement for a putative neuroprotective drug because of their diverse pharmacological activities.”
LikeLike