|
In December of of 2017, the results of a clinical trial suggested that a particular kind of exercise may have beneficial effects against certain aspects of Parkinson’s. Specifically, a high-intensity treadmill regime was found to be ‘non-futile’ as an intervention for the motor symptoms in de novo (newly diagnosed) Parkinson’s. Recently, however, new pre-clinical research has been published which reported that when mice with particular Parkinson’s-associated genetic mutations are exercised to exhaustion, they have high levels of inflammation which can exaggerate the neurodegeneration associated with that model of PD. So naturally, some readers are now asking “So should I be exercising or not?!?” In today’s post we will review the results of the two studies mentioned above, and discuss why exercise is still important for people with Parkinson’s.
|
Readers are recommended to click on the image above and listen to the music (Michael Sembello’s “Maniac” from 1983) whilst reading this post.
This song was made famous by one particular scene from the 1983 movie “Flashdance” starring Jennifer Beals, in which the lead character undertook an intense dance routine. Ever since that iconic scene, exercise fanatics have long used the music to help get themselves into the mood for their workouts.
————————
One of my personal life goals. Source: Jobcrusher
Few experts would disagree that the benefits of exercise are many.
Adults who achieve at least 2.5 hours of physical activity per week have:
- up to a 35% lower risk of coronary heart disease and stroke
- up to a 50% lower risk of type 2 diabetes
- up to a 50% lower risk of colon cancer
- up to a 20% lower risk of breast cancer
- a 30% lower risk of early death
- up to an 83% lower risk of osteoarthritis
- up to a 68% lower risk of hip fracture
- a 30% lower risk of falls (among older adults)
- up to a 30% lower risk of depression
- up to a 30% lower risk of dementia
(Source: NHS)
But what about people with PD? What do we know about exercise and Parkinson’s?
In November 2017, a useful (and very thorough) review article was published by researchers from Hong Kong on the long-term effects of exercise on people with Parkinson’s:
Title: Long-term effects of exercise and physical therapy in people with Parkinson disease.
Authors: Mak MK, Wong-Yu IS, Shen X, Chung CL.
Journal: Nat Rev Neurol. 2017 Nov;13(11):689-703.
PMID: 29027544
In this review, the authors focused their attention solely on the long-term effects of exercise (studies of >6-12 months). This review only included studies with a training period lasting at least 12 weeks with a minimum of 12 weeks of follow-up after treatment has ended.
In their review of the literature, they found:
- Most progressive strength and aerobic endurance training programmes have positive effects that last for 12 weeks
- Extended progressive strength training improves muscle strength for up to 24 months and aerobic endurance training increases walking capacity at 6–16 months
- Balance training improves balance, gait and mobility, and reduces falls for up to 12 months after completion of treatment
- Gait training improves gait performance and walking capacity for up to 6 months after training
- Tai chi and dance improve balance and tai chi reduces fall frequency up to 6 months after training
- A training period of at least 6 months is effective for achieving clinically meaningful improvement in UPDRS-III scores
Now, all of that said, the review did make some important observations associated with these findings:
- For people with early-stage Parkinson’s (that is recently diagnosed PD), training enabled the reduction of their amount of L-dopa treatment (Click here to read more about this).
- A reduction of falls was found only in patients with mild Parkinson’s (UPDRS motor score ≤26 – click here to read more about this).
- People with advanced Parkinson’s (Hoehn and Yahr stage ≥2.9) generally failed to maintain exercise-driven gains in gait speed at their follow-up assessments (Click here to read more about this).
These findings led the authors of the review to conclude that severity of Parkinson’s “seems to have an effect on treatment outcome” of exercise. And they proposed that if there were to be any clinical evaluation of exercise on people with Parkinson’s – to determine if it could potentially be used as a treatment for delaying disease progression – it would be best to target recently diagnosed individuals. This would be the best cohort to first assess whether exercise could have any effect on PD.
Has there been a proper clinical trial of exercise in early-stage Parkinson’s?
Yes there has.
In December of 2017, the results of a Phase II clinical study were published in journal JAMA Neurology:
Title: Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De NovoParkinson Disease: A Phase 2 Randomized Clinical Trial
Authors: Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, Josbeno DA, Christiansen CL, Berman BD, Kluger BM, Melanson EL, Jain S, Robichaud JA, Poon C, Corcos DM.
Journal: JAMA Neurol. 2017 Dec 11. doi: 10.1001
PMID: 29228079
In this study – called the “Study in Parkinson’s Disease of Exercise” (or SPARX) – the researchers wanted to “examine the feasibility and safety of high-intensity treadmill exercise in patients with de novo Parkinson disease who are not taking medication and whether the effect on motor symptoms warrants a phase 3 trial” (Click here for more information about this clinical study). De novo meaning ‘recently diagnosed’.
The SPARX study only recruited individuals with idiopathic Parkinson’s (Hoehn and Yahr stages 1 or 2), aged between 40 to 80 years, within 5 years of diagnosis, and who were not expected to required any L-dopa medication for the entire 6 months of the study.
It was a Phase II, multi-center clinical trial in which participants were randomly assigned to 3 groups:
- A high-intensity treadmill exercise (4 days per week, 80%-85% maximum heart rate) - 43 participants
- A moderate-intensity treadmill exercise (4 days per week, 60%-65% maximum heart rate) - 45 participants
- A ‘usual care’ group (who were instructed to maintain normal exercise habits) - 40 participants
Treadmill exercise. Source: Powerhouse-fitness
The average age across all of the groups was 63-64 years of age (and for the purposes of the discussion further below please note this average age). On all other demographics and baseline measures the three groups were incredibly well balanced for randomised groupings.
The treadmill exercise was conducted 4 days per week for 26 weeks, and it involved:
- 5 to 10 minutes of warm-up
- 30 minutes of treadmill exercise at the target heart rate
- 5 to 10 minutes of cool down.
Exercise frequency/intensity were gradually increased during weeks 1 to 8 until the target levels were reached, and from there on the target heart rate was maintained by adjusting treadmill (speed and/or incline). All of the participants used a heart rate monitor to record the intensity of all their exercise sessions. At the beginning of the study (weeks 1-2), all sessions were supervised at the study site.
The primary outcome of the study was the 6-month change in the Unified Parkinson’s Disease Rating Scale (UPDRS) Part III motor score, and throughout the study the clinical assessors were blind to which group each participant belonged to.
So what were the results of the study?
The ‘moderate-intensity’ treadmill exercise group exhibited no difference when compared with the ‘usual care’ group at 6 months for all of the UPDRS measures (not just motor scores).
BUT, the average change in UPDRS motor score in the ‘high-intensity’ group was +0.3 (range: −1.7 to 2.3) compared with +3.2 (range: 1.4 to 5.1) in the ‘usual care’ group (the average change in the ‘moderate-intensity’ group was +2.0 (0.38-3.7)
Thus, the researchers concluded that high-intensity treadmill exercise led to less motor change compared with usual care.
And this allowed them to state that high-intensity treadmill exercise was nonfutile and warranted further investigation.
Nonfutile?
Yeah, I know. Silly word.
Source: Youtube
Basically, the study suggested that high-intensity treadmill exercise, 3-4 times per week appears to slow the progression of the motor decline in people with recently diagnosed Parkinson’s.
High-intensity treadmill exercise was not futile.
Wow! That’s great!
Well, yes.
But one small ‘rain-on-the-parade’ note here: There was no difference between the high-intensity treadmill exercise group and the usual care group at the 6 months assessment on any of the other UPDRS scores (for example, cognition, etc).
The effect was limited to just the motor scores.
Ok, but still, this is an impressive result!
Yes it is. And everyone got very excited with the news.
The researchers still need to see replication of the effect in a larger Phase III clinical study of course, but this probably hasn’t stopped many folks with Parkinson’s rushing to take out an annual gym membership, and hitting the treadmills with great intensity.
Source: Anytimefitness
So summing up, what does it all mean?
Uh no, not quite.
You see, recently there has been some lab-based research which has taken some of the excitement out of the treadmill study results. And the SoPD has fielded quite a few concerned emails about this new research.
In August, this report was published:
Title: Parkin and PINK1 mitigate STING-induced inflammation
Authors: Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, Burman JL, Li Y, Zhang Z, Narendra DP, Cai H, Borsche M, Klein C & Youle RJ
Journal: Nature, 2018, Sep;561(7722):258-262.
PMID: 30135585
And in this study, the researchers wanted to determine if inflammation contributes to or is a consequence of neuronal loss that is characteristic in Parkinson’s.
What is inflammation?
Inflammation is an immune response to an unexpected situation in the body (for example, injury or infection).
The function of inflammation is to eliminate the initial cause of the problem (such as a damage causing pathogen like a virus), clear out dying cells and tissues damaged from the original insult, clear out dying cells and tissues damaged by the inflammatory process itself, and initiate tissue repair.
And the researchers used a genetically engineered mouse which exhibits inflammation due to mitochondrial stress.
What is mitochondrial stress?
Regular readers of this blog are probably getting sick of the picture below.
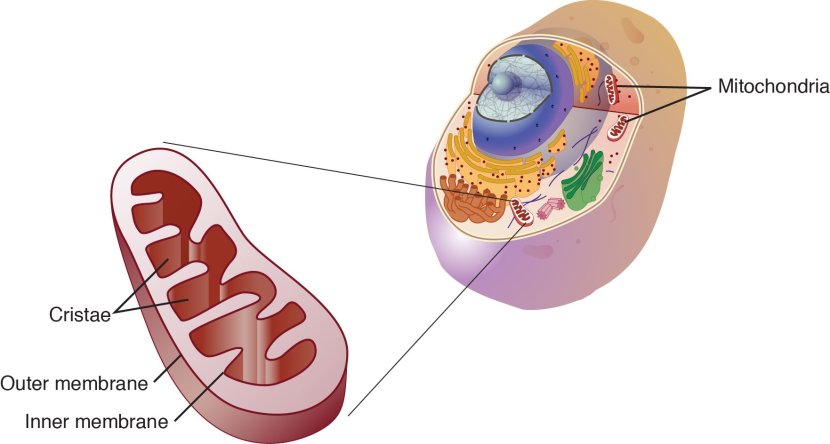
Mitochondria and their location in the cell. Source: NCBI
I use it quite often on this website, because it nicely displays a basic schematic of a mitochondrion (singular), and where mitochondria (plural) reside inside a cell.
Mitochondria are the power stations of each cell. They help to keep the lights on. Without them, the party is over and the cell dies.
Ok, but where does the ‘stress’ part come into ‘mitochondrial stress’?
Well, like you, me and all other things in the universe, mitochondria each have a use-by date.
With all their busy power production work, mitochondria get old and worn out (or damaged) with time. When mitochondria becomes sick and needs to be disposed of, they will begin to exhibit mitochondrial stress – the release of messenger molecules that state very clearly “these mitochondria need to be disposed of”. I say ‘very clearly’ because if left unattended, these mitochondria and their messenger molecules will make the cell very sick and ultimately kill it.
A cell will dispose of the mitochondria via a process called mitophagy (a blending of the words mitochondria and autophagy – the waste disposal system of each cell).
How does mitophagy work?
Removal of old/damaged mitochondria via mitophagy involves two steps:
- Induction of general autophagy, and
- Priming (or labelling) of the mitochondria for autophagic recognition
Let’s start with the first step, because that is the easy part. General autophagy is conducted inside a phagophore. A phagophore is a double membrane bag that forms and encloses around objects inside a cell that need to be disposed of. The phagophore isolates the rubbish until it can be broken down. The formation of the phagophore occurs in a step wise fashion, as is illustrated in this image below which displays the autophagic removal of a mitochondria:
Mitophagy. Source: Circres
Once the phagophore has matured and fully enclosed the rubbish, the phagophore then waits to fuse with a lysosome. A lysosome is a bag of enzymes that break things like mitochondria down into tiny components that can either be re-used or disposed of outside the cell.
Ok, and what about the “priming of the mitochondria for autophagic recognition” step you mentioned above?
The second step.
Within a cell, there needs to be a system of identifying old or dysfunctional parts. This is done by a process of labelling (or priming) the material that is going to be disposed of. Without such a system, rubbish would simply build up and phagophore would not know what to form around.
How is this labelling/priming done?
Mother nature has taken no chances with the important process of waste disposal, and there are multiple ways that the labelling can occur. For example, in the image below there are three of methods presented (A, B & C) for labelling mitochondria for disposal (and the phagophore can form – blue arc on the right):
The pathways to mitophagy. Source: AJPheart
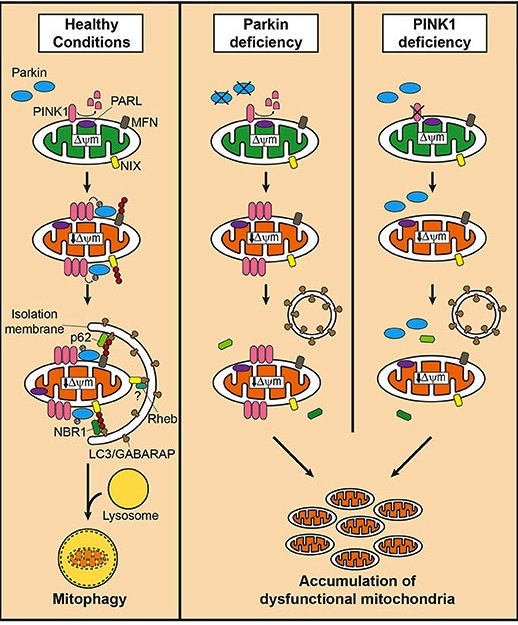
And key amongst these various methods for our discussion today is the first one which involves two proteins called PTEN-induced putative kinase 1 (or PINK1) and PARKIN.
What is special about them?
Well the researchers who were interested in investigating inflammation and mitochondrial stress in this second study, used mice that were genetically engineered to have either no PINK1 protein or no PARKIN protein.
These mice represent a model of Parkinson’s.
How?
Approximately 20% of Parkinson’s cases are associated with particular variations in DNA that render people vulnerable to developing the condition (Click here to read more on the genetics of Parkinson’s). Some of these variations are in specific regions of DNA (called genes) that provide the instructions for making particular proteins.
There are currently 23 genes that are referred to as ‘PARK genes’, as genetic variations within those genes make people more vulnerable to developing Parkinson’s.
Two of those PARK genes (PARK2 and PARK6) are involved in mitophagy.
And can you guess which two proteins these two genes provide the instructions for building???
That is right: PARKIN and PINK1 (respectively)
What do PINK1 and PARKIN do?
Both proteins appear to have many different functions, but their roles in the process of mitophagy are relatively well understood.
PINK1 acts like a kind of handle on the surface of mitochondria. In normal, healthy cells, the PINK1 protein attaches to the surface of mitochondria and it is slowly absorbed until it completely disappears from the surface and is degraded. In unhealthy cells, however, this process is inhibited and PINK1 starts to accumulate on the outer surface of the mitochondria. Lots of handles poking out of the surface of the mitochondria.
Now, if PINK1 is a handle, then PARKIN is a flag that likes to hold onto the PINK1 handle. While exposed on the surface of mitochondria PINK1 starts grabbing the PARKIN protein. This pairing is a signal to the cell that this particular mitochondrion (singular) is not healthy and needs to be removed. The pairing start the process that leads to the development of the phagophore and eventually mitophagy.

PINK1 and PARKIN in normal (right) and unhealthy (left) situations. Source: Hindawi
In the absence of normal PINK1 or PARKIN proteins, there is no handle-flag system and old/damaged mitochondria start to pile up and exhibit mitochondrial stress. They are not disposed of appropriately and as a result the cell gets sick and ultimately dies.
Mitophagy. Source: Frontiersin
People with particular genetic mutations in the PINK1 or PARKIN genes are vulnerable to developing an early onset form of Parkinson’s (generally before 40 years of age – again, please note the age). It is believed that the dysfunctional disposal of (and accumulation of) old mitochondria are part of the reason why these individuals develop the condition at such an early age.
Ok, so these researchers are interested in looking at inflammation in mice with no PINK1 or PARKIN?
Exactly.
But the researchers noticed that markers of inflammation in the mice were not much different than the PINK1 or PARKIN mutant mice when compared to normal mice. So the investigators began to wonder if they needed to stress the mice in order to generate a difference in markers of inflammation.
The researchers did this by putting the mice through ‘forced exhaustive treadmill running‘ – basically the mice were run until they couldn’t run no more. Following this exhaustive exercise, the researchers noticed that the PINK1 or PARKIN mutant mice had very high levels of markers of inflammation when compared to the normal mice. And these high levels were maintained 2 days after the exhaustive exercise session (returning to normal levels at 6 days post exercise).
Interesting side note here – the researchers also tested mice with the Parkinson’s associated LRRK2 (G2019S) genetic variant, and did not see this difference in high levels of markers of inflammation. The high levels of inflammation after exhaustive exercise were specific to the PINK1 or PARKIN mutant mice.
The researchers found that by treating the PINK1 or PARKIN mutant mice with an inhibitor which blocked an inflammation associated molecule (called interferon receptor), they were able to reverse this increase in high levels of markers of inflammation immediately following exhaustive exercise. This treatment returned markers of inflammation back to levels observed in normal mice.
Source: Pinterest
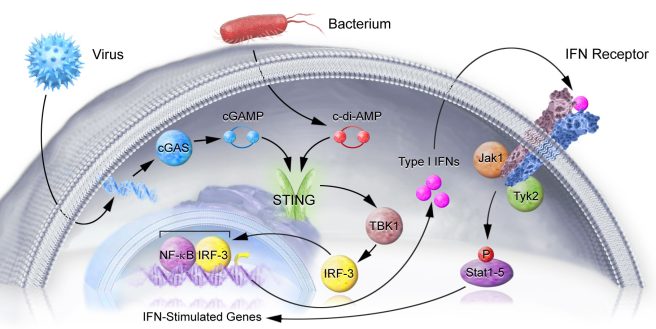
Now, since blocking interferon receptor was able to reduce levels of inflammation, the researchers started looking at a protein called STING.
What is STING?
Stimulator of interferon genes (or STING) is critically involved with the production of the inflammatory marker interferon, which occurs when cells are infected with some nasty pathogen (a disease causing agent).
STING is a signalling molecule that sits on the outer surface of the endoplasmic reticulum – the part of the cell where proteins are produced. The activation of STING leads to the production of Interferon (Type I IFNs in the image below), which then exit the cell, and bind to the cell’s own interferon (IFN) receptor. And activation of the IFN receptor leads to the activation of pro-inflammatory genes.
The STING pathway. Source: Nimbus
It is a round-about kind of process, but it helps to cause only affected cells to alert the immune system that something is wrong – Click here for a good open access review of STING.
So what did the researchers do with STING?
They genetically engineered mice that had no STING protein.
And those mice were ok?
Yep. As happy as Larry (that’s a Kiwi/Aussie phrase). The mice were fine – running around, doing what mice do.
Ok. Then what happened?
The researchers bred the PARKIN and PINK1 mutant mice with the ‘no STING’ mice, resulting in PARKIN or PINK1 mutant mice with the ‘no STING’ protein. These mice either produced no PARKIN and STING proteins, or they produced no PINK1 and STING proteins.
And those new mice were ok?
Yep. As happy as Larry.
And then…?
And then the researchers tested these new mice (no PARKIN + no STING; or no PINK1 + no STING) on their exhaustive exercise experiment – they ran them until they could not run any more.
And guess what?
These new mice exhibited NO very high levels of markers of inflammation at the end of their exercise session. Even when compared to the normal mice, the markers of inflammation in these “no PARKIN + no STING” or “no PINK1 + no STING” mice looked perfectly normal.
The researchers also found that blocking STING was able to reduce the level of dopamine cell loss observed in the brains of PARKIN mutant mice (resulting from inflammatory signals from mitochondrial stress). And this finding suggested to the researchers that inflammation may be facilitating this process. They hypothesise that “PARKIN and PINK1 prevent inflammation and neurodegeneration by clearing damaged mitochondria,….suggesting a new model for how mitophagy may mitigate Parkinson’s”
This was all in mice. Did the researchers look at humans?
Yes, they did.
The researchers looked at markers of inflammation in blood samples collected from people with idiopathic (or spontaneous; cannot be explained by genetics) Parkinson’s and from people with PINK1 or PARKIN genetic variations.
They found that people with idiopathic Parkinson’s and PARKIN genetic variants have high levels of markers of inflammation in their blood (compared to controls). Curiously, individuals with PINK1 genetic variants did not differ from controls in their levels of markers of inflammation in their blood.
This difference suggests that therapies inhibiting STING could be of interest to certain members of the Parkinson’s community.
Is anyone looking at inhibiting STING in humans?
Yes, there are biotech companies currently trying to develop STING inhibitors (or antagonists).
One example of this is Nimbus Therapeutics.
The company is focused on developing small-molecule STING antagonists (inhibitors) for inflammatory conditions like in lupus and other interferonopathies. Last year (2017), Nimbus entered a long-term strategic alliance with the much larger biotech company Celgene Corporation to accelerate this process.
And they are not alone in the search of STING inhibitors – another biotech company called IFM Therapeutics is also developing them.
IFM Therapeutics was bought by the pharmaceutical company Bristol-Myers Squibb in 2017, and they are continuing to develop antagonists of the STING pathway.
When these companies have STING antagonists ready for clinical testing, it may be interesting to have a look at whether these compounds could be useful in Parkinson’s (do they cross the blood brain barrier?).
But what does all this mean with regards to my exercise routine?
Ok, so now we get to the business end of things with the question on everyone’s lips. This new ‘STING’ research report has led to some readers not being ‘as happy as Larry’ and to subsequently worry about whether their exercise regime may not be as beneficial as first hoped.
On the one hand, we have research saying high intensity exercise is potentially slowing the decline of motor ability in people with Parkinson’s, but on the other hand, we have a report suggesting that exhaustive exercise may be detrimental in particular genetics-based forms of Parkinson’s.
What to do? What to do?
Ok, firstly, all of the results in this post need to be independently replicated before we start to draw any major conclusions. If you are concerned, however, it would be best to talk to your doctor/clinician about this situation.
Second, please do NOT start popping anti-inflammatories after each gym session before you speak with your doctor. Some anti-inflammatories can have undesirable side-effects (such as gastrointestinal bleeding). In addition, taking anti-inflammatory drugs (such as Advil and ibuprofen) after a workout will result in slower recovery times (Click here and here to read more about this).
Third, if the results can be replicated, we may be looking at a potential novel avenue of therapy for the Parkinson’s community (particularly those with a PARKIN variant).
I have a PARKIN genetic variation. Should I exercise?
As I say, the results need to be independently verified before anyone starts to panic. We see a lot of research reports that make a big splash with a major result, only to find that no one can replicate them later on. So lets wait till there is more information available on this.
I am not a medical clinician and I do not like to provide much advice here, but until we see that further research on this matter, perhaps ease up on the ‘exhaustive exercise’ (e.g. running until you can’t run anymore) and rest well after other exercise. Also: eat healthy (avoid inflammatory foods – source).
So what does it all mean?
I have struggled since starting this website to provide a post on exercise and Parkinson’s.
People are always asking for information about it – because it is an area of the condition where they can be proactive and feel that they have a level of control – but I have had a very difficult time pulling together all of the research and finding a.) significant results that have been demonstrated under carefully controlled conditions, and b.) something that is immediately applicable for members of the community (for example, no special equipment required beyond the standard stuff you find in a gym).
Thus, when the clinical trial results were published in December last year, I thought I had found the study to base this post around. In addition, the very useful review article (Mak et al, Nature Reviews Neurology, 2017 Nov, 13(11):689-703 – which I discussed above) was published a few weeks before the clinical study and that provided a lot of additional material. ‘Great’ I thought, ‘smooth sailing from here on’.
Then along came the hurricane STING.
A lot of folks (particularly those with a PARKIN variant) got upset by this report – and naturally so. But it is important that we see the results replicated before we read too much into it. In addition, if the results are replicated, this could be very useful information – for example, pointing us in specific directions for potentially treating individuals with PARKIN genetic variations in particular.
But we need to wait and see what the follow up results suggest.
EDITORIAL NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. Dietary changes can impact the effectiveness of treatments. PLEASE speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Picturequotes













Thanks – for the honest review
LikeLike
Thanks Don – hope all is well.
LikeLike
This article really helped me to understand how PARKIN and PINK1 mutations can lead to PD. And also, how mitochondria are disposed of within cells.
But unless I missed it, there is one loose end in this research: what about the buildup of excess mitochondria?
This was described as harmful, in and of itself, and as something that ultimately leads to cell death. And yet, at a certain point, the focus shifted to the inflammatory process that *results* from the buildup of undisposed dysfunctional mitochondria. It was found that disabling STING prevented that inflammation.
OK, so with no STING, we no longer have inflammation, but we still have dysfunctional mitochondria building up, right? So how come the problem appeared to be solved, then? Is the *only*, or at least *main* negative effect of dysfunctional mitochondrial buildup the inflammatory response that it invokes?
The researchers are like, “well, we disabled the inflammatory response by taking away STING, hooray, now we have a new drug target.” And, indeed, the mice seemed restored to their usual mousy selves through that remedy. But are they going to hit a wall at some point further down the road, because the dysfunctional mitochondria are continuing to accumulate?
And, come to think of it, maybe the reason that the non-stressed PARKIN / PINK1 mice did not exhibit inflammation is because their mitochondria were not wearing out as *fast*, and so there were fewer dysfunctional ones that needed disposal. And maybe if the experiment had gone on long enough, those lazy mice would *also* have developed problems due to the more *gradual* buildup of dysfunctional mitochondria.
LikeLike
Am I the only one wishing for some elaboration on the definition of “usual care” (the group that fared the best in the aforementioned “High-Intensity Treadmill Exercise” study)?
LikeLiked by 1 person
Hi Simon,
I cannot let a superb study on exercise go by without saying thanks for the information. The experimental outcomes seemed to show recently-diagnosed PWP got more out of exercise than grizzled vets like me (16 years of symptoms and counting!) . In particular it was pointed out that advancing years and PD saw the effects of hard exercise decrease as training DRIVE ran down. True science is a numbers game and my solitary experience adds up to nothing BUT it works for me – 45 years of hard exercising and the more I push it the better my body behaves – I felt this more so than ever before last week when I was so busy sorting out final touches to a forum that I missed 4 days out of 7. Okay, I thought no problem I will easily get back into it in a couple of weeks time but I was wrong! My mobility suffered a bit and my balance was almost shot during regular off periods each day. By the end of that week I was struggling to keep going so, in order to be able to keep working on the forum I wound the exercise rubber band up and pounded my way through workouts and my movement and balance has improved quite a bit in just a few days.
I am convinced that the most important factor in exercise is attitude – take on the struggle as though you were taking on someone in the ring- and give it hell.
Nearly all my past training partners and colleagues have given up or train much easier routines now but think they are working hard and the evidence is there to see when a long-term Parky-person (me) can out-perform them. Like a computer if we input a lot of work we get more output. Yes we get older but the majority of work capacity loss comes from working less intensely as our lazy minds try to talk us out of pushing and suffering. We need to be able to endure- as Bruce Lee said ‘Do not pray for an easy life, pray for the strength to endure a hard life.’ He was quite a philosopher!
LikeLike
My limited experience is similar- more exercise equals less symptoms. FWIW
LikeLike
The SPARX study measured UPDRS scores which essentially means it measured symptoms.
The STING study measured biological markers which plausibly means it measured things much closer to causes.
Could it not be the case that high intensity exercise makes the cause worse even while it hides the fact by making the symptoms better?
LikeLike