|
Lewy bodies are densely packed, circular clusters of protein that have traditionally been considered a characteristic feature of the Parkinsonian brain. Recently, however, evidence has been accumulating which calls into question this ‘defining feature’ of the condition. The presence Lewy bodies in some cases of other neurological conditions (such as Alzheimer’s), and their complete absence in some cases of Parkinson’s, are leading many researchers to question their pivotal role in PD. In today’s post, we will look at a new research report of Parkinson’s post mortem cases studies which present no Lewy bodies, and we will disucss what this might mean for our understanding of Parkinson’s and the future treatment of the condition.
|
 Neuropathologists conducting a gross examination of a brain. Source: NBC
Neuropathologists conducting a gross examination of a brain. Source: NBC
At present, a definitive diagnosis of Parkinson’s can only be made at the postmortem stage with an examination of the brain. Until that moment, all cases of Parkinson’s are ‘suspected’. When a neuropathologist makes an examination of the brain of a person who passed away with the clinical features of Parkinson’s, there are two characteristic hallmarks that they will be looking for in order to provide a final diagnosis of the condition:
1. The loss of specific populations of cells in the brain, such as the dopamine producing neurons in a region called the substantia nigra, which lies in an area called the midbrain (at the base of the brain/top of the brain stem). As the name suggests, the substantia nigra region is visible due to the production of a ‘substance dark’ molecule called neuromelanin in the dopamine neurons. And as you can see in the image below, the Parkinsonian brain has less dark pigmented cells in the substantia nigra region of the midbrain.
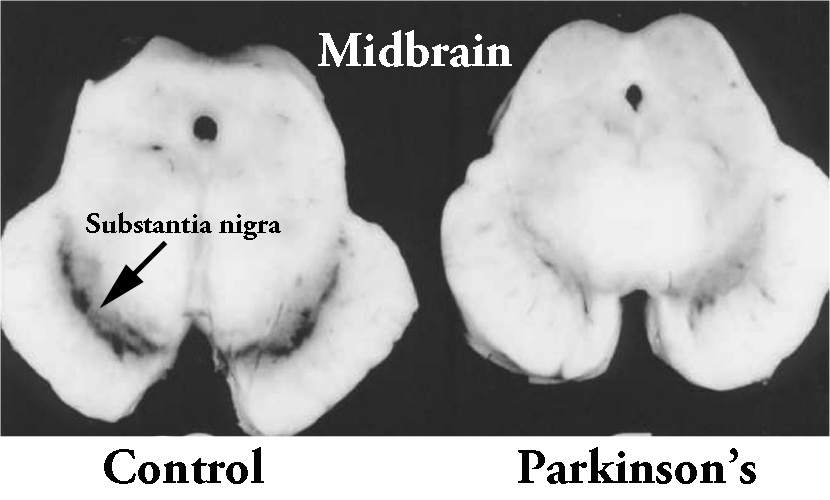 The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
2. Dense, circular clusters (or aggregates) of protein within cells, which are called Lewy bodies.
 A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
A Lewy body is referred to as a cellular inclusion, as they are almost always found inside the cell body. They generally measure between 5–25 microns in diameter (5 microns is 0.005 mm) and thus they are tiny. But when compared to the neuron within which they reside they are rather large (neurons usually measures 40-100 microns in diameter).
 A photo of a Lewy body inside of a neuron. Source: Neuropathology-web
A photo of a Lewy body inside of a neuron. Source: Neuropathology-web
Do all Parkinson’s brains have Lewy bodies?
This is a really interesting question. Welcome to the topic of this post.
Here’s the thing about Lewy bodies: They used to be considered the ‘Parkinson’s feature’, but now they are everywhere.
In 1986, researchers noticed that they were present in a high proportion of the brains of people with Alzheimer’s:

Title: Parkinson’s disease in patients with Alzheimer’s disease.
Authors: Leverenz J, Sumi SM.
Journal: Arch Neurol. 1986 Jul;43(7):662-4.
PMID: 3729742
Of the 40 Alzheimer’s brains that these researchers looked at nearly half of them (18 cases) had either dopamine cell loss or Lewy bodies – the characteristic features of Parkinsonian brain – in a region called the substantia nigra (where the dopamine neurons are located). They next went back and reviewed the clinical records of these cases and found that rigidity, with or without tremor, had been reported in 13 of those patients. According to their analysis 11 of those patients had the pathologic changes that warranted a diagnosis of Parkinson’s.
And the most surprising aspect of this research report: Almost all of the follow up studies, conducted by independent investigators found exactly the same thing!
As a result, it is now generally agreed by neuropathologists (the folks who analyse sections of brain for a living) that 20% to 50% of cases of Alzheimer’s have the characteristic round, cellular inclusions that we call Lewy bodies which are typically associated with Parkinson . In fact, in one analysis of 145 Alzheimer’s brains, 88 (that is 60%!) had verified Lewy bodies (Click here to read more about that study).
And it doesn’t stop there.
Even normal healthy individuals (without Parkinson’s or Alzheimer’s) can have Lewy bodies.
Excuse me?
There was the study which involved the analysis of 273 brains from people who died from conditions other than Parkinson’s. The researchers in that study found that the presence of Lewy bodies in the ‘normal’ brain increased from 3.8% of cases to 12.8% between the sixth and ninth decades – and remember these brains were not associated with cases of Parkinson’s (Click here to read about that research).
Another study found that 33 of 139 brains (or 23%) from ‘clinically normal’ individuals contained Lewy bodies in various regions (Click here to read that study).
But Lewy bodies are present in all cases of Parkinson’s though. Right?
Actually, no.
And this week a report was published which provides a good example of the absence of Lewy bodies in some cases of Parkinson’s:
Title: Isolated nigral degeneration without pathological protein aggregation in autopsied brains with LRRK2 p.R1441H homozygous and heterozygous mutations
Authors: Takanashi M, Funayama M, Matsuura E, Yoshino H, Li Y, Tsuyama S, Takashima H, Nishioka K, Hattori N.
Journal: Acta Neuropathol Commun. 2018 Oct 17;6(1):105.
PMID: 30333048 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers report on two Japanese families in which ten individuals have developed a slowly progressing, late-onset parkinsonism. An analysis of DNA from seven of those affected individuals indicates that they have mutations (or variants – errors in their DNA) in a very specific region of DNA called PARK8.
What is PARK8?
Your DNA can be divided into short functional regions (which are called genes). Some of these genes provide instructions for the production of proteins. There are approximately 23 genes where variations have been found that are associated with an increased risk factors for developing Parkinson’s.
These genes are referred to as PARK genes.

An early list of the PARK genes. Source: JKMA
PARK8 is one of these PARK gene, and it is also known as Leucine-rich repeat kinase 2 (or LRRK2 – pronounced ‘lark 2’).
What is LRRK2?
Also known as ‘Dardarin‘ (from the Basque word “dardara” which means “trembling”), LRRK2 is an enzyme that has many functions within a cell – from supporting efforts to move things around inside the cell to helping to keep the power on (involved with mitochondrial function).

The many jobs of LRRK2. Source: Researchgate
The gene that provides the instruction for making the LRRK2 enzyme is made up of many different sections, each of which is involved with the different functions of the eventual protein.
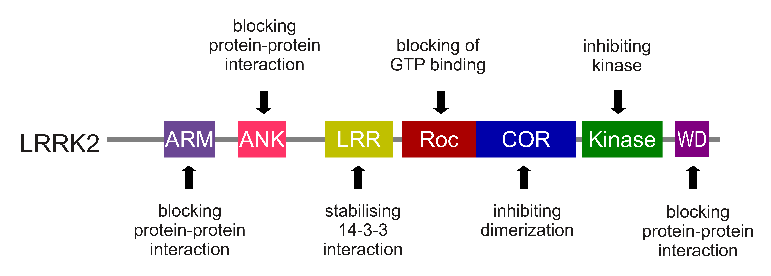
The regions and associated functions of the LRRK2 gene. Source: Intechopen
Genetic variations within the LRRK2 gene are recognised as being some of the most common with regards to increasing ones risk of developing Parkinson’s. But despite being one of the ‘most common’ genetic risk factors, variants within the LRRK2 gene are only present in approximately 1-2% of all cases of Parkinson’s. They are rather rare.

The structure of Lrrk2 and where various mutations lie. Source: Intech
As the image above suggests, mutations in the PARK8 gene are also associated with Crohn’s disease (Click here and here for more on this) – though that mutation is in a different location to those associated with Parkinson’s. And one particularly common Parkinson’s-associated LRRK2 mutation – called G2019S – is also associated with increased risk of certain types of cancer, especially for hormone-related cancer and breast cancer in women – Click here to read more about this. If you have a G2019S mutation, no need to panic, it is good to be aware of this association and to have regular check ups with your doctor.
The G2019S mutation (the name designates its location on the gene) is the most common LRRK2 mutation. In some populations of people it can be found in 40% of people with Parkinson’s (Click here to read more about this). But (as I mentioned above) in general, LRRK2 mutations are rare within the PD community.
What is interesting about the G2019S mutation is that it gives rise to a LRRK2 enzyme that is hyperactive.
The structure of LRRK2 protein. Source: Wikipedia
As a protein, LRRK2 interacts with many different types of other proteins, and you can imagine that in a finely balanced environment like the cells that a mutant hyperactive form of LRRK2 is going to cause problems. And it is this hyperactive behaviour of the LRRK2 protein that is believed to be involved with the cellular dysfunction seen in LRRK2-associated Parkinson’s.
Another hotspot’ of mutations in the LRRK2 gene is the ROC region (the red domain in the image below):

The structure of Lrrk2 and where various mutations lie. Source: Intech
And the Japanese families mentioned in today’s research report have a genetic mutation in this region, which is called LRRK2 p.R1441H. This variant results in “a twofold reduction in GTPase activity and a twofold increase in GTP-binding affinity” – don’t worry about what that means, but the output of this activity is (again) a hyperactive version of the LRRK2 protein (Click here to read more about this).
These hyperactive versions of LRRK2 have been the focus of a great deal of research – some of which is out of this world (Click here to read more about this) – and pharmaceutical companies have been rushing to develop LRRK2 inhibitors with the goal of using them to treat people with Parkinson’s (Click here to read more about this).
Ok, so these two Japanese families affected by Parkinson’s have a genetic variation (LRRK2 p.R1441H) in their LRRK2 gene, which causes hyperactive LRRK2 protein?
Yes, and this has resulted in a slow progressing, late-onset form of Parkinson’s.
But here’s the thing: the researchers have obtained post mortem samples of brains from three of the 10 affected individuals, and guess what?
They found NO LEWY BODIES.
The researchers did observe a significant loss of dopamine neurons in the substantia nigra (the region where the dopamine producing cells live). In the image below you can see an age matched control sample in panel A with lots of tiny brown dots (the dopamine neurons), and a lot less in panels B-C which provide images from the 3 affected members of the Japanese familes:
 Source: Actaneurocomms
Source: Actaneurocomms
So, the dopamine neurons were being lost.
But when the researchers looked for the presence of Lewy bodies, they found none. In the image below you can see an example of Lewy bodies (brown dots and lines labelling alpha synuclein protein) in panel E, but very few if any in panels F-H:
 Source: Actaneurocomms
Source: Actaneurocomms
(I am not entirely clear on what the brown dots in Panel H are, but the researchers state that phosphorylated alpha synuclein was ‘not detected at all in the brainstem, limbic area, subcortical nuclei, white matter, or neocortex in any’ of the three cases)
The investigators conclude that this particular PARK8/LRRK2 p.R1441H mutation results in dopamine neuron degeneration without protein aggregation. When they reviewed previous reports of postmortem brain analyses from other PARK8/LRRK2 mutation cases, dopamine neuron degeneration was the constant finding across all cases. Evidence of protein aggregation/Lewy bodies was inconsistent (but please note that those studies involved different LRRK2 genetic variants).
They concluded that the neuronal cell loss in these cases was caused by neurotoxicity of the mutant LRRK2 protein, rather than any actual protein aggregation. Therefore, if protein aggregation was occurring in these cases, it was occuring downstream to the hyperactive activity of LRRK2.
Is this the first time anyone has not found Lewy bodies in the brains of people with Parkinson’s?
No, it is not.
Previous post mortem studies looking at Parkinsonian brains with different LRRK2 genetic mutations have also reported dopamine neuron degeneration without Lewy bodies. Those cases have included the p.G2019S, p.R1441G, and p.I2020T mutations (Click here and here to read more about this).
And loss of dopamine neurons with no sign of Lewy bodies has also been observed in the brains of some people with other Parkinson’s associated genetic variants, such as PARKIN and PINK1 (Click here to read more about this).
So you can hopefully now see why some researchers are questioning the validity of Lewy bodies as the ‘definitive’ characteristic of the PD brain.
So what does it all mean?
Every week we are learning more and more about Parkinson’s. And each bit of replicated/validated data is not only another piece in the puzzle, but also potentially indicative of where things may head in the future – treatment-wise.
This week, researchers have provided postmortem evidence suggesting that individuals with Parkinson’s and a particular genetic variation in a Parkinson’s associated gene (LRRK2), have no Lewy bodies in their brains. While this information is interesting from a biological standpoint, it may also have important implications for how we will treat this form of Parkinson’s in the future.
There are ongoing clinical trials which are attempting to remove the toxic version of alpha synuclein as it is floating around in the brain (Click here to read a previous SoPD post on this topic). But if some individuals do not have alpha synuclein-filled Lewy bodies in their brains, we have to ask whether such a treatment (called immunotherapy) would actually be beneficial for them.
Luckily, there are other potential therapeutic approaches being developed by researchers and biotech companies which may well work in these cases, such as LRRK2 inhibitors (Click here to read more about this). And given that the mutations can be deteched early in life, it could be very interesting in the future to test whether such treatments could actually slow or prevent PD from ever developing in the future generations that carry this LRRK2 mutation.
Thus, while our understanding of the condition shifts, so to do our future treatment options.
The banner for today’s post was sourced from NPR


Seems rather important, thanks. Maybe no lewy bodies over here,
“There are ongoing clinical trials which are attempting to remove the toxic version of alpha synuclein as it is floating around in the brain. But if some individuals do not have alpha synuclein-filled Lewy bodies in their brains, we have to ask whether such a treatment (called immunotherapy) would actually be beneficial for them.”
LikeLike
Fascinating and confusing, of course. And the topsy turvy scientific upending of ‘well known’ characteristics of PD might be amusing, if it didn’t affect a PwP like me personally. Simon, might you know if there are any phenotypic differences in PwP with versus WITHOUT Lewy bodies, perhaps related to presence versus absence of specific nonmotor symptoms? Also, where does this leave the Braak hypothesis of staged spreading of Lewy body pathology? As you imply at the end of your blog, when we move back to thinking about subtyping PD, doesn’t presence or absence of Lewy bodies (and it’s complicated, if it’s not an all-or-none phenomenon) suggest that this might be a target of important subtyping, for both treatment trials and longitudinal observations of disease course ?
LikeLike
Great post Simon! Were they drinking green tea (EGCG)?
LikeLike
Simon, here we have a classic case for the (urgent) need to formalize PD sub-typing. If the current a-syn immunotherapy trials don’t take this variable into account, they could easily mistake a lack of response to the drug (by non-Lewy-body PD individuals) as failure of the drug in those individuals – thereby lowering the sensitivity of the trial.
LikeLike