|
In this end-of-year post we review the year that was 2018. Month-by-month we will briefly discuss some of the major pieces of research/announcement that have define the year and advanced our understanding of Parkinson’s. The list is based on nothing more than the author’s personal opinion – apologies to any researchers who feel left out. And in the next post we will consider what the year ahead (2019) has in store for us.
|
 Source: a-star
Source: a-star
In the 525600 minutes that made up 2018, a lot happened in the world of Parkinson’s research.
A total of 7672 research papers were published with the keyword ‘Parkinson’s’ according to the Pubmed website (this compared to 7675 for all of 2017 – this obviously represents a dismal failure for the Parkinson’s research community: the first time in quite a while that we haven’t beaten the number of research reports from the previous year!
 I am of course kidding. The quantity of research reports is irrelevant. But it does make me smile that we missed the mile stone by just 3 papers!
I am of course kidding. The quantity of research reports is irrelevant. But it does make me smile that we missed the mile stone by just 3 papers!
2018 has been another amazing year for Parkinson’s research. And while I appreciate that a comment like this means little to someone living with the condition on a day-to-day, remarkable progress has been made not only in our understanding of the condition, but also in the various ways in which the research is being done and potential therapies are approaching the condition.
In this post, we will review the year that was by briefly summarising some of the major research-related events of each month in 2018.
And that journey begins with:
In January, Voyager Therapeutics announced FDA clearance to begin dosing participants for their pivotal Phase 2-3 clinical trial of VY-AADC for advanced Parkinson’s. This is a gene therapy approach for PD (Click here to read the press release and click here to read a SoPD post on this topic).
We also got the first in vivo evidence that Parkinson’s associated PINK1 is detectable at basal levels. Analysis of endogenous levels suggest that loss of PINK1 does not influence basal mitophagy (Click here to read the research report). This result was verified a few days later by another independent lab (Click here to read the preprint manuscript).
During January, researchers reported that postmortem Parkinson’s brain samples display increased astrocytic senescence. They also observed that cultured human astrocytes exposed to the pesticide paraquat become senescent in cell culture. And they found that clearance of senescent cells mitigates cell loss in a mouse model of PD (Click here to read the research report and click here for a SoPD post on this topic).
And finally, miniaturized neural drug delivery system (or MINDS) are new cannulas (made up of several tubes, each approx. 30 micrometers in diameter), that can be implanted in the brain and can deliver different types of medications. Implications for Parkinson’s? Interesting possibilities for this: – multiple drugs could be administered (or tested: think adaptive clinical trials) – no blood brain barrier issues – targeted to specific brain structures – limited off-target peripheral side effects. Verdict: Very cool! (Click here to find out more).
 In February, researchers presented evidence of what may be Lewy bodies in mice. Lewy bodies are one of the defining features of the Parkinsonian brain, but we have very rarely ever observed them in models of Parkinson’s. New research suggests that the loss of autophagy (part of the waste recycling system) in dopaminergic neurons causes Parkinson’s-like Lewy bodies to appear, as well as dopamine cell loss and motor dysfunction similar to Parkinson’s in aged Atg7 conditional knockout mice. If independently validated, these mice could represent a fantastic new tool for testing novel PD-focused therapies as well as drugs for repurposing (Click here to read the research report and click here to read a SoPD post on this research).
In February, researchers presented evidence of what may be Lewy bodies in mice. Lewy bodies are one of the defining features of the Parkinsonian brain, but we have very rarely ever observed them in models of Parkinson’s. New research suggests that the loss of autophagy (part of the waste recycling system) in dopaminergic neurons causes Parkinson’s-like Lewy bodies to appear, as well as dopamine cell loss and motor dysfunction similar to Parkinson’s in aged Atg7 conditional knockout mice. If independently validated, these mice could represent a fantastic new tool for testing novel PD-focused therapies as well as drugs for repurposing (Click here to read the research report and click here to read a SoPD post on this research).
 A Lewy body inside a mouse brain. Source: Nature
A Lewy body inside a mouse brain. Source: Nature
In addition, researchers have found that the element calcium binds to one end of the Parkinson’s-associated protein alpha-synuclein which increases its lipid-binding capacity. They also found that calcium & alpha synuclein levels mediate dopamine toxicity and that the calcium channel blocker ‘Isradipine’ rescued cells from that toxicity – this is good news as Isradine is currently being clinically tested for Parkinson’s (Click here to read more about the research and click here and here to read more about the clinical trial).
And research looking at the structure of the LRRK2 protein after it was grown in low gravity orbit failed to provide better results than those achieved on Earth – back to the drawing board with this idea. A better understanding of the structure of LRRK2 will allow for better LRRK2-targeting drugs to be designed (Click here to read the manuscript and click here to read a SoPD post on this topic).
 Spring brought new research suggesting that as Parkinson’s associated alpha‐synuclein protein aggregates, it activates the calcium pump SERCA leading to calcium dysregulation. Pharmacological inhibition of SERCA protects cells from alpha synuclein aggregate stress which provides the research community with novel avenues for therapeutic intervention (Click here to read this research report and click here for a SoPD post on the topic).
Spring brought new research suggesting that as Parkinson’s associated alpha‐synuclein protein aggregates, it activates the calcium pump SERCA leading to calcium dysregulation. Pharmacological inhibition of SERCA protects cells from alpha synuclein aggregate stress which provides the research community with novel avenues for therapeutic intervention (Click here to read this research report and click here for a SoPD post on the topic).
 Source: EMBO
Source: EMBO
In March, researchers identified a conformationally distinct species of the Parkinson’s-associated protein alpha synuclein. It is present in neuronal cultures & mice brains exposed to α-synuclein fibrils AND in the postmortem brains of people who passed away with Parkinson’s. The results of the research suggest that alpha-synuclein undergo autophagic degradation (waste recycling), but this process is incomplete. This leads to the formation of a form of alpha synuclein that the researchers called Pα-syn*. These Pα-syn* aggregates exit the lysosomes and then localise to mitochondria where they start to cause trouble. The researchers conclude that “pα-syn* is a major neurotoxic species inducing mitochondrial damage, fission, & mitophagy, therefore constituting a central player in PD pathogenesis”(Click here to read the abstract of this research and click here to read a SoPD post on the topic).
And inhaled anaesthetics suddenly became an area of research for the PD community. Inhaled anaesthetics have long been known to have biological effects beyond simply sedating individuals. Some of those effects are beneficial, while others….mmm, well, not so beneficial. New research from Toronto suggests that inhaled anaesthetics may induce neuronal protein aggregation and also affect endoplasmic reticulum trafficking. The endoplasmic reticulum is a structure closely aligned with the nucleus in a cell, and it is critically involved with protein synthesis. The researchers do not look at Parkinson’s-associated proteins (such as alpha synuclein or Tau) directly, but there are interesting potential implications (Click here to read the research report and click here to read a SoPD post on the topic).
 In April, researchers reported that NKCC1 chloride importer antagonist, Bumetanide, provides a novel therapeutic strategy for Parkinson’s based on a restoration of low chloride levels which corrects GABAergic inhibition and improves motor ability in a mouse model of Parkinson’s. Bumetanide is a clinically available drug used to treat heart failure. Some side effects (exacerbates diabetes & gout). The researchers are attempting to start a phase II clinical trial for Bumetanide in Parkinson’s following a small pilot study (Click here to read the research report, click here to read more about that pilot study, and click here to read a SoPD post on the topic).
In April, researchers reported that NKCC1 chloride importer antagonist, Bumetanide, provides a novel therapeutic strategy for Parkinson’s based on a restoration of low chloride levels which corrects GABAergic inhibition and improves motor ability in a mouse model of Parkinson’s. Bumetanide is a clinically available drug used to treat heart failure. Some side effects (exacerbates diabetes & gout). The researchers are attempting to start a phase II clinical trial for Bumetanide in Parkinson’s following a small pilot study (Click here to read the research report, click here to read more about that pilot study, and click here to read a SoPD post on the topic).
April also found new associations between Inflammatory bowel disease, Parkinson’s and anti-TNF therapy. Researchers report that individuals with inflammatory bowel disease (IBD) have 28% higher risk of developing Parkinson’s. They also found a lower rate of PD among people with IBD who were exposed to anti-TNF therapy vs those not (Click here to read more about the research and Click here to read a SoPD post on the topic).
And finally, researchers found that a fish-derived protein called β-parvalbumin readily inhibits the formation of the aggregated form of Parkinson’s-associated alpha synuclein. The researchers naturally ask if “fish intake may provide health benefits” for people with Parkinson’s (Click here to read the full report and click here for an SoPD post on this topic).
 The month of May began with an update on the vaccine of Parkinson’s. Austrian biotech company AFFiRiS has announced long-term data from their first-in-human clinical studies of AFFITOPE PD01A which targets the toxic (oligomeric) form of alpha synuclein in early Parkinson’s. The vaccine was found to be safe and well tolerated for the 4 year period of the study. In addition the press release suggested that ‘clinical scores were stable for the entire study period’, though study not designed for efficacy (Click here to read the research report and click here to read a SoPD post on this topic).
The month of May began with an update on the vaccine of Parkinson’s. Austrian biotech company AFFiRiS has announced long-term data from their first-in-human clinical studies of AFFITOPE PD01A which targets the toxic (oligomeric) form of alpha synuclein in early Parkinson’s. The vaccine was found to be safe and well tolerated for the 4 year period of the study. In addition the press release suggested that ‘clinical scores were stable for the entire study period’, though study not designed for efficacy (Click here to read the research report and click here to read a SoPD post on this topic).
There was also news of different types of cells in the brain having different types of protein aggregates.The Parkinson’s-associated alpha synuclein clusters (or aggregates) in glial cells are conformationally & biologically distinct from the Lewy bodies found in neurons. Different strains of alpha synuclein protein are determined by both misfolded seeds AND intracellular environments (Click here to read more and click here to read the press release).
In addition, UK researchers found that Parkinson’s‐associated LRRK2 affects innate immune control of tuberculosis infections. The researchers found that by reducing levels of LRRK2, they reduced the rate at which TB could infect cells and animals. Unexpected result perhaps, but as a result, LRRK2 inhibitors which are being developed for Parkinson’s could also be tested on TB (1/4 of world pop. infected) (Click here to read more about the research, click here to read the press release, and click here to read a SoPD post on the topic).
 June was a really big month for Parkinson’s research in 2018. Researchers reported that increasing NAD+ levels (via the NAD+ precursor nicotinamide riboside) significantly reduces mitochondrial issues in cells from people with GBA-associated Parkinsons. In addition, nicotinamide riboside treatment prevents the age-related dopaminergic neuronal loss & motor decline in fly models of GBA-associated (Click here to read more about this, click here to read the press release, and click here for a SoPD post on the topic).
June was a really big month for Parkinson’s research in 2018. Researchers reported that increasing NAD+ levels (via the NAD+ precursor nicotinamide riboside) significantly reduces mitochondrial issues in cells from people with GBA-associated Parkinsons. In addition, nicotinamide riboside treatment prevents the age-related dopaminergic neuronal loss & motor decline in fly models of GBA-associated (Click here to read more about this, click here to read the press release, and click here for a SoPD post on the topic).
In addition, researchers reported that flies with Parkinsons-associated PARKIN & PINK1 genetic mutations have circadian & sleep defects. Treating them with the phospholipid Phosphatidylserine helps to rescue those defects. (Click here to read more about this, click here to read the press release, and click here for an SoPD post on this topic).
There was also news of a new brain-penetrant Glucagon-like peptide-1 (GLP1) receptor agonist, called NLY01, being prepared for clinical testing. It protected against the loss of dopaminergic neurons & behavioral deficits in models of Parkinson’s, by blocking microglial-induced conversion of astrocytes to an A1 toxic state (Click here to read more about this and Click here to read an SoPD post on this topic and click here for another interesting commentary about this research).
AND a new c-Abl inhibitor, called Radotinib, which may be better than Nilotinib which is currently being tested in the clinic. Researchers found that Radotinib is neuroprotective in a preclinical model of Parkinson’s. Apparently better pharmacokinetic properties & safety profiles than Nilotinib! Developed by South Korean firm Ilyang Pharmaceutical, Radotinib is currently in phase III testing for chronic myeloid leukemia. Prof Ko & co seem very keen to clinically test Radotinib in Parkinson’s and related α-synucleinopathies (Click here to read the research report and click here to read a SoPD post on this topic).
And finally, researchers have identified Trodusquemine (an aminosterol similar to squalamine) as an inhibitor not only of the initial phases of Parkinson’s-associated alpha synuclein aggregation but also the fibril-dependent secondary pathways. This result is particularly interesting because of the ability of trodusquemine to cross the blood-brain barrier (Squalamine does not!). “The present results suggest that this compound has the potential to be an important therapeutic candidate for Parkinson’s & related disorders” (Click here to read more about this and click here to read a SoPD post on this topic).
 With July came news of a new role for the adaptive immune system in Parkinson’s. Researchers found an increase in a particular type of immune cell (T-cells) in post mortem section of brain from people with PD. They also found a high level of one particular type of T-cell (IL-17-producing helper T-cells) in PD blood (compared to healthy control sample). These T-cells induce cell death in cell culture. Blocking of IL-17 or the addition of the FDA-approved anti-IL-17 antibody, secukinumab, rescued the neuronal death – Secukinumab is FDA approved for the treatment of moderate-to-severe plaque psoriasis (Click here to read more about this and click here to read a SoPD post on this topic).
With July came news of a new role for the adaptive immune system in Parkinson’s. Researchers found an increase in a particular type of immune cell (T-cells) in post mortem section of brain from people with PD. They also found a high level of one particular type of T-cell (IL-17-producing helper T-cells) in PD blood (compared to healthy control sample). These T-cells induce cell death in cell culture. Blocking of IL-17 or the addition of the FDA-approved anti-IL-17 antibody, secukinumab, rescued the neuronal death – Secukinumab is FDA approved for the treatment of moderate-to-severe plaque psoriasis (Click here to read more about this and click here to read a SoPD post on this topic).
Researchers also reported that, independent of any mutations, Leucine-rich repeat kinase 2 (or LRRK2) may play a role in idiopathic Parkinson’s. Meaning: the LRRK2 kinase inhibitors being developed could be useful for treating PD in people without LRRK2 mutations (Click here to read more about this, Click here to read a press release about this research, and click here to read an SoPD post on this topic)
August brought new data regarding the genetics of Parkinson’s. Researchers made available a report outlining a study in which they analysed DNA from 37,700 cases of Parkinsons & 1.4 million (!?!) controls and find 92 genetic risk factors for PD – 39 of them completely novel! Analysis of biological pathways associated with the data finds “6 annotations were related to vacuolar functionality and autophagy, 3 pathways for endosomal trafficking, 2 pathways for catabolism related functions, & 2 lysosomal pathways”. Despite all of this new data, however, these 92 genetic risk factors only account for less than 20% of cases of Parkinson’s (Click here to read more about this and click here to read a SoPD post on this topic).
Researchers also reported that the removal of a protein called ‘Stimulator of interferon genes’ (or STING) reduces inflammation mediated by mitochondrial stress and protects mice with Parkinson’s assoicated genetic mutations (PINK1 or PARKIN). The researchers report that a strong inflammatory state in both PARKIN mutant mice and PINK1 mutant mice following exhaustive exercise (the mice ran until they could not run any more). Inflammation resulting from the exhaustive exercise was completely rescued by concurrent loss of STING. An interesting target for novel therapies? (Click here to read more about this and click here for an editorial on this report).
And in silico drug screening for PD (using genome-wide association study data) by researchers identifies 57 FDA approved drugs that could be useful for Parkinson’s. These drugs included Dabrafenib – an anti-melanoma drug. The investigators then demonstrated that Dabrafenib could rescue both cell & mouse models of Parkinson’s. This result is interesting, because there is a curious connection between Parkinson’s and melanoma: People with Parkinson’s in general have a reduced risk of developing all known cancers except one – take a wild guess which one? People with Parkinson’s are 4 to 8 times more likely to have melanoma than people without Parkinson’s. So it is interesting that a melanoma-targeting drug displays beneficial effects in models of Parkinson’s (Click here to read more about this and click here for the press release).
 In September, a research report was published which involved large consortium of academic and biotech researchers who conducted a HUGE screening experiment to identify compounds that markedly inhibit the clustering (or aggregation) of Parkinson’s-related alpha synuclein. They screened 746,000 compounds – yes, 3/4 of a million compounds and identified 58 compounds that reduce aggregation as well as 100 compounds that increase it. Most effective aggregation inhibitors were derivatives of (4-hydroxynaphthalen-1-yl)sulfonamide. But of particular interest to us was the positive control compounds they used: Epigallocatechin Gallate (or EGCG) and baicalein (Click here to read more about this, and click here and here to read SoPD posts on this study).
In September, a research report was published which involved large consortium of academic and biotech researchers who conducted a HUGE screening experiment to identify compounds that markedly inhibit the clustering (or aggregation) of Parkinson’s-related alpha synuclein. They screened 746,000 compounds – yes, 3/4 of a million compounds and identified 58 compounds that reduce aggregation as well as 100 compounds that increase it. Most effective aggregation inhibitors were derivatives of (4-hydroxynaphthalen-1-yl)sulfonamide. But of particular interest to us was the positive control compounds they used: Epigallocatechin Gallate (or EGCG) and baicalein (Click here to read more about this, and click here and here to read SoPD posts on this study).
New research was published suggesting that people with Attention Deficit Hyperactivity Disorder (or ADHD) may be twice as likely as members of the general public to go on to develop Parkinson’s-like conditions. And this risk increases to 4x normal rates in individuals with ADHD who were treated with stimulant medication. The researchers found that of the 31,769 people with ADHD, only 56 went on to develop Parkinson’s (0.18% of the total group), compared to 96 (0.06%) of the control group (non-ADHD; 158,790 individuals). And of the 4,960 records of people with ADHD who were prescribed stimulant medications in this study, only 19 were subsequently diagnosed with PD (Click here to read more about this and click here to read a SoPD post on this topic).
 October started off with a bang! The results of the Phase Ib (Prothena) clinical trial of PRX002/RG7935 – an anti–alpha synuclein monoclonal antibody – in people with Parkinson’s were published. 80 participants, observed over 24 weeks, looks safe & well tolerated. The Phase II efficacy trial is ongoing (Click here to read more about these results, Click here to read a SoPD post on the topic, and Click here to read an interesting editorial on the results).
October started off with a bang! The results of the Phase Ib (Prothena) clinical trial of PRX002/RG7935 – an anti–alpha synuclein monoclonal antibody – in people with Parkinson’s were published. 80 participants, observed over 24 weeks, looks safe & well tolerated. The Phase II efficacy trial is ongoing (Click here to read more about these results, Click here to read a SoPD post on the topic, and Click here to read an interesting editorial on the results).
 In addition, we learnt about the potential benefits of not having an appendix. A new report found that the human appendix contains an abundance of misfolded alpha synuclein protein and that removal of the appendix decreased one’s risk of developing Parkinson’s. Using two independent epidemiological datasets (involving more than 1.6 million individuals), the researchers observed that removal of the appendix several decades before the onset of Parkinson’s was associated with a lower risk for PD, particularly for people living in rural areas, and it delayed the age of PD onset (Click here to read more about this, click here and here to read the press releases, and click here to read a SoPD post on this topic).
In addition, we learnt about the potential benefits of not having an appendix. A new report found that the human appendix contains an abundance of misfolded alpha synuclein protein and that removal of the appendix decreased one’s risk of developing Parkinson’s. Using two independent epidemiological datasets (involving more than 1.6 million individuals), the researchers observed that removal of the appendix several decades before the onset of Parkinson’s was associated with a lower risk for PD, particularly for people living in rural areas, and it delayed the age of PD onset (Click here to read more about this, click here and here to read the press releases, and click here to read a SoPD post on this topic).
 There was also exciting news regarding how inflammasome inhibition prevents alpha synuclein pathology. Researchers have found that, in mouse models of Parkinson’s, alpha synuclein protein aggregates promotes the activation of inflammatory processes (called the inflammasome) in the immune cells of the brain: the microglia. The researchers also report that an oral treatment – a small-molecule NLRP3 inhibitor, called MCC950 being developed by a biotech company called Inflazome – improved motor performance and reduced neuroinflammation, neurodegeneration, and alpha synuclein accumulation in mouse models of Parkinson’s (Click here to read more about this, click here to read the press release, and click here to read a SoPD post on this topic).
There was also exciting news regarding how inflammasome inhibition prevents alpha synuclein pathology. Researchers have found that, in mouse models of Parkinson’s, alpha synuclein protein aggregates promotes the activation of inflammatory processes (called the inflammasome) in the immune cells of the brain: the microglia. The researchers also report that an oral treatment – a small-molecule NLRP3 inhibitor, called MCC950 being developed by a biotech company called Inflazome – improved motor performance and reduced neuroinflammation, neurodegeneration, and alpha synuclein accumulation in mouse models of Parkinson’s (Click here to read more about this, click here to read the press release, and click here to read a SoPD post on this topic).
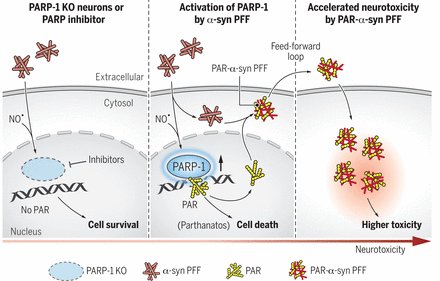
I don’t like to give any opinions on this website, but in November a research paper was published that I think deserves the title ‘Parkinson’s research paper of the year’. In the report, the researchers demonstrated that Parkinson’s-associated alpha synuclein aggregates activate poly(adenosine 5′-diphosphate–ribose) (PAR) polymerase–1 (PARP-1), which leads to parthanatos cell death. PARP inhibitors rescue mouse Parkinson’s models. The report suggests alpha synuclein is killing cells by activating PARP (via the parthanatos cell death pathway). PARP activation leads to increased levels of poly(adenosine 5′-diphosphate–ribose) (PAR) which goes on to accelerate alpha synuclein aggregation (in a feed-forward cycle). And the investigators also demonstrate that PAR levels are increased in the brains of people with Parkinson’s. In addition, they found that treating with clinically available PARP inhibitors significantly reduced levels of PAR in models of PD, which in turn resulted in less alpha synuclein aggregation. The only thought here is that PARP inhibitors are not great at crossing the blood brain barrier, so translating these results to people with Parkinson’s will be challenging (Click here to read more about this, click here to read a commentary, click here to read the press release, and click here to read a SoPD post on this topic).
Researchers also published an impressive report using high-resolution, single-cell transcriptomic analyses of patient-derived dopamine neurons (made from induced pluripotent stem cells or iPSCs) to identify histone deacetylase 4 (HDAC4) as a regulator of disease progression. Treating the iPSC-derived dopamine neurons with HDAC4-modulating compounds like Tasquinimod – an allosteric inhibitor of HDAC4 – corrected Parkinson’s-related cellular phenotypes. Another potential drug for repurposing (Click here to read more about this).
In December, a pair of research papers published at the same time basically stole the show. Researchers demonstrated that stearoyl-CoA desaturase inhibition rescues alpha synuclein toxicity and neuron degeneration in models of Parkinson’s. The investigators were interested in what happens with lipids in a situation of high levels of alpha synuclein. They found that by reducing levels of unsaturated membrane lipids – via the inhibition of the oleic acid-generating enzyme ‘stearoyl-CoA-desaturase’ – in human neurons, they could protect the cells from alpha synuclein toxicity & enhance their survival. Both excessive alpha synuclein or genetic variation associated with Parkinson’s (such as SCNA triplication) appears to alter lipid homeostasis in human neurons grown in cell culture. Stearoyl-CoA-desaturase inhibition decreases alpha synuclein inclusions and increases alpha synuclein tetramer:monomer ratio. One of the senior researchers on this paper – Prof Susan Lindquist – passed away in 2016, but such was her contribution to the field of Parkinson’s & neurodegenerative research that here she is still publishing as we approach 2019 (Click here and here to read more about this, click here for the press release, and click here for a SoPD post on this topic).
In December, we also recieved some disappointing news: On the 10th December, the National Institute of Health recommended the early closure of the Phase III clinical study of Inosine in Parkinson’s, due to failure to achieve the primary end point of the study (demonstrating efficacy in slowing disease progression – Click here to read the press release about this).
But in keeping with the balance in the universe, we also recieved some very good news: Acorda Therapeutics announced that the FDA has finally approved INBRIJA – an inhalable form of L-dopa – for the treatment of “OFF” times in Parkinson’s. The therapy is designed to rapidly alleviate symptoms. This also represents the first treatment directly funded by the Michael J Fox Foundation to come to market (Click here to read more about this and click here for an old SoPD post on this topic).
And those were the research highlights/announcements from 2018.
In the next post we will be looking ahead to 2019 and what the new year may hold for the Parkinson’s community.
Until then, enjoy your happy new year parties and try to stay out of trouble.
SoPD score card for 2018 – Totals: 110 posts; 362,281 words
The banner for today’s post was sourced from Strategicvisionpr



































I really appreciate all this. My wife was diagnosed three years ago and I keep her in touch with all the optimistic stuff coming out of the research community. It really helps. Having attended talks from Roger Barker I’d be interested to understand the state of play on stem cell research, if you have time? Thank you
LikeLike
Hi Mark,
Thanks for your comment – glad you liked the post. Sorry to hear of your wife’s diagnosis, I hope the medication are making the situation easier.
I have recently put up a post that looks at the year ahead and it that post we discuss the state of play on stem cell research – https://scienceofparkinsons.com/2019/01/10/2019/. Please let me know if you have any other questions regarding the topic.
Kind regards,
Simon
LikeLike
This as always great and I will now spend a lot of time reading. Just wanted to ask if you have anything new on the MCC950 molecule.
LikeLike
Re; MCC950-H had my dates wrong Last posting Brain on Fire was current.
LikeLike
Hi Dale,
Thanks for your comments – I am watching MCC950 and several other NLRP3 inhibitors closely. I will let you know when there is any news.
Kind regards,
Simon
LikeLike
More on this – here is an interesting overview of the current efforts to target the inflammasome in Parkinson’s:
https://www.nature.com/articles/d41587-019-00005-8
LikeLike
Dear Simon,
Thank you for this summary, and the summary updates throughout 2018. My husband was diagnosed early in 2018, and your posts (along with the links to other excellent reference sources) have helped both of us along a steep learning curve. Your posts consistently break down the topics with clear explanations of medical terms and accompanying diagrams – I still find a lot of it hard to understand, but your posts really help explain the context of each finding, and the potential implications for individuals with degenerative diseases.
LikeLike
Hi Paula,
Thanks for your comment and kind words – I’m pleased you find the website useful. Please let me know if there are any topics in particular that you would like better discussed/explained.
Kind regards,
Simon
LikeLike
Hi Simon,
I’d like to second Mark’s request for a state-of-play regarding stem-cell research. I’ve seen the “mini state-of-play” within the “2019 preview” post, but it would be great to also get your thoughts on the use of mesenchymal stem cells (MSCs).
There are now three clinical trials involving the intravenous injection of MSCs to treat Parkinson’s disease. One trial is using bone-marrow-derived MSCs (NCT02611167 – Texas) and the other two are using umbilical-cord-tissue-derived MSCs (NCT03684122 – Jordan,NCT03550183 – China).
Many thanks, and best wishes for 2019.
Jeff
LikeLike
Hi Jeff,
Thanks for your comment, but I need to be careful how I answer this as I do not wish for my words to be taken out of context.
There is a lot of research being conducted on Parkinson’s, in many different areas. And there is a very human tendency to ‘go with the herd’ (follow the popular trends) within the research world. This can of course result in personal biases within individual researchers. That said, if I am completely honest, I am yet to be convinced by the mesenchymal stem cell research in Parkinson’s. And I say that coming from a background in fetal & embryonic stem cell work, where preclinical restoration has a rather long history.
In addition, while I have great confidence in the ability of the researchers conducting the MSC studies (particularly the Texas group), there are a lot of direct-to-consumer outfits that are selling MSC-based therapies that have not been clinically tested or proven. I do not wish for any positive comment here regarding MSC studies to be used out of context by any unscrupulous operators, but nor do I want the serious research-led clinical trials to be stigmatised or affected by associations to the direct-to-consumer shops.
All of this said, I will be very interested to see the results of the Texas MSC trial when they finish later this year.
I hope you understand my position.
Kind regards,
Simon
LikeLike
Hi Simon,
Thanks for the detailed reply. Yes, I can understand your caution.
Also, I have not yet exhausted the power of Google, so I’m still a happy searcher.
Warm Regards,
Jeff
LikeLike
For anyone who may be interested, here is (some of?) the preclinical research underpinning the Texas MSCs-for-PD clinical trial.
https://www.researchgate.net/publication/277977887_A_Meta-Analysis_of_Mesenchymal_Stem_Cells_in_Animal_Models_of_Parkinson's_Disease
LikeLike