|
Glial cell-line derived neurotrophic factor (or GDNF) has been a topic of heated discussion in the Parkinson’s community for a long time. Most recently due to the announcement of the results of the Phase II Bristol GDNF clinical trial results, which did not meet the primary end points of the study (Click here to read more about that). This week at the annual American Association of Neurological Surgeons conference in San Diego, the results of another GDNF clinical trial were presented. This new study was a Phase I study assessing the safety and tolerability of a gene therapy approach for GDNF in people with Parkinson’s. In today’s post, we will discuss what gene therapy is, what the new trial results indicate, and what the researchers may be planning to do next for this new clinical trial programme.
|
 Source: AANS
Source: AANS
Every year members of the American Association of Neurological Surgeons gather together in one spot and compare data/research/clinical notes.
This year the 87th AANS Annual Scientific Meeting was held in spectacular San Diego.
 San Diego. Source: AFP
San Diego. Source: AFP
From Saturday 13th April through till Wednesday 17th, clinicians and researchers attended lectures and discussed new data on every aspect of neurological surgery. While I did not (nor planned to) attend the meeting, I was very interested to learn more about one particular presentation.
It involved the announcement of the results of a clinical trial which was focused on a gene therapy approach for Parkinson’s.
The treatment involved GDNF (Click here to read the abstract).
What is GDNF?
GDNF stands for glial cell line-derived neurotrophic factor.
Glial cells are the support cells in the brain. While neurons are considered to be the ‘work horses’ of neurological function – passsing messages and storing memories – glial cells are in the background making sure that neurons are supported, protected and nurtured.
There are different types of glial cells, including astrocytes, oligodendrocytes and microglia. And each type has a specific function, for example microglia are the brain’s resident immune cells checking up on the health of the neurons while oligodendrocytes provide the neurons with a protective covering (called myelin sheath) which also helps to speed up the signalling of neurons.
 Different types of cells in the brain. Source: Dreamstime
Different types of cells in the brain. Source: Dreamstime
Astrocytes provide nutrients to neurons and make sure the environment surrounding the neurons is balanced and supportive. Glial cells are absolutely critical to the normal functioning of the brain.
The researchers that discovered GDNF found this protein in a cell culture of rat glial cells – hence the name: glial cell-line derived.
So that is the “glial cell-line” part of glial cell line-derived neurotrophic factor, now let’s focus on the latter part.
Neurotrophic factors (neurotrophic = Greek: neuron – nerve; trophikós – pertaining to food/to feed) are chemicals that nurture neurons and support growth. There are many types of neurotrophic factors, some having more beneficial effects on certain types of neurons and not other.
GDNF is one of these neurotrophic factors.
Of particular interest to us is that GDNF is very neuroprotective for dopamine neurons (Click here for a very good OPEN ACCESS review of GDNF biology). Dopamine neurons are one group of cells in the brain that are badly affected by Parkinson’s. Thus, any protein that protects them and stops them from dying is of great interest to the Parkinson’s research community.
Ok, and what is meant by gene therapy?
Gene therapy is an experimental treatment approach that involves treating medical conditions with DNA rather than drugs.
Gene therapy basically involves introducing a new piece of DNA or replacing a faulty piece of DNA within a population of cells. DNA, as you may remember from high school science class provides the instructions for making proteins in the a cell and these proteins are the bits that actually do stuff.
By introducing a new piece of DNA into a cell, the cell can start to produce a functioning protein that it may not normally produce. In some diseases, a cell may normally produce a particular protein, but because the genetic instructions in the DNA (a section of the DNA called a gene) for that protein have a small error (a genetic mutation), a non-functioning version of the protein is actually being produced. The introduction of the new correct (functioning) version of that piece of DNA (or gene) into a cell can start the production of a functional version of the protein.
 Gene therapy. Source: yourgenome
Gene therapy. Source: yourgenome
Alternatively, a gene can be introduced into a cell which would cause the cell to produce a protein that that cell usually does not produce. This sort of approach is being used in gene therapy for cancer, where ‘suicide genes’ are being introduced into cancer cells. These cause the cancer cell to die, by initiate an auto-destruct sequence resulting in cell death (a process called apoptosis). Another approach the cancer field is using is introducing a gene into cancer cells that cause a protein to be produced on the surface of the cancer cell that attracts the attention of the immune system. This ‘marker gene’ causes the immune system to attack the cancer cell, resulting in the death of the cancer cell.
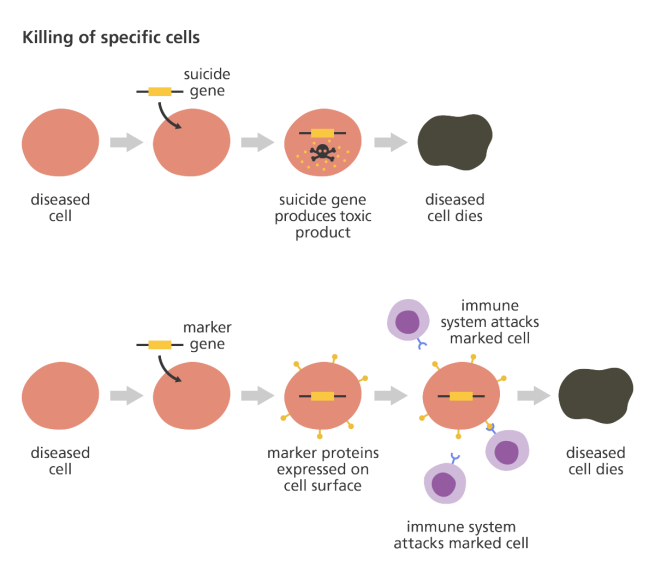 Source: yourgenome
Source: yourgenome
Taking this approach one step further, we can take sections of DNA that contain the genes involved with the production of a proteins that would be beneficial for the cell, such as GDNF. By then injecting a virus with the DNA for GDNF into the brain, we can produce GDNF in any infected cells (it’s slightly more complicated than that, but you get the basic idea).

Gene therapy for Parkinson’s disease. Source: Wiki.Epfl
I’m sorry, but did you say viruses?
Yes, if you remove the viral DNA from inside a virus and replace it with something useful, then a virus becomes a very useful biological delivery system. Far superior to anything we humans have devised thus far. Viruses are easy to produce and manipulate, and they can even be engineered to target specific cell types.
And these viruses have been engineered not to replicate. They deliver the proposed DNA and that is all.
I see. Has this gene therapy approach ever been tested in models of Parkinson’s?
Yes it has. Many times in fact.
Almost immediately after the discovery of GDNF was announced, researchers began trying to stick the DNA of GDNF into empty viruses with the goal of infecting cells in the brain and causing them to produce the protein. The first successful demonstration of this feat in a model of Parkinson’s was published in 1997:
 Title: Dopaminergic neurons protected from degeneration by GDNF gene therapy.
Title: Dopaminergic neurons protected from degeneration by GDNF gene therapy.
Authors: Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC.
Journal: Science. 1997 Feb 7;275(5301):838-41.
PMID: 9012352
In this study, the researchers inserted the DNA for GDNF into an adenovirus, and injected it into the part of the brain where the dopamine neurons reside (the substantia nigra). This treatment resulted in a 3 fold reduction in the loss of dopamine neurons 6 weeks after a neurotoxin (6-OHDA) was delivered (compared with no gene therapy or an empty virus control treatment).
And this initial GDNF-based gene therapy result was replicated by multiple independent labs very quickly (Click here and here for other examples).
But the type of virus used in gene therapy is important. Adenoviruses are known to cause immune responses in mammals. And this has caused researchers to shift to AAV viruses.
What are AAV viruses?
Adeno-associated viruses (or AAV) are a kind of virus that are popular with researchers because A.) they readily infect human and primate cells, and B.) they produce little (if any) immune response, and C.) they are non-pathogenic (they don’t cause any known diseases).
Given these characteristics, AAVs have been used in most of the gene therapy clinical trials thus far:
 AAV-based gene therapy clinical trials. Source: Wikipedia
AAV-based gene therapy clinical trials. Source: Wikipedia
They were originally discovered in the preparation of another type of virus, called an adenovirus (hence the name ‘Adeno-associated’). They were believed to simply be a contaminant of that preparation. Further research, however, revealed that AAVs belong to the Dependoparvo genus of viruses, which in turn belongs to the family Parvoviridae.
AAVs are single-stranded DNA viruses, and they are one of the smallest viruses (approximately 22 nm in diameter) with a non-enveloped capsid. The capsid is the shell surrounding the genetic material of the virus. Viruses are either enveloped or non-enveloped. “Enveloped” means that a second casing surrounds the capsid, providing further protection for the virus, while “non-enveloped” viruses have only the capsid.
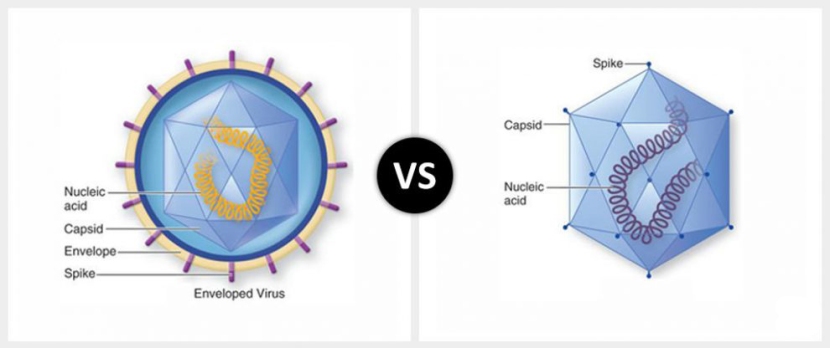
Enveloped (left) vs Non-enveloped (right) viruses. Source: Differencebtwn
Given the reduced amount of casing, non-enveloped viruses are generally more virulent (more infectious) than enveloped viruses (a good example of a non-enveloped virus is the influenza virus). Non-enveloped viruses do not survive outside of an organism for long though.

The AAV capsid. Source: Wikipedia
And AAV viruses have been found to be very effective at delivering GDNF DNA into models of Parkinson’s, for example:
 Title: Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats.
Title: Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats.
Authors: Mandel RJ, Spratt SK, Snyder RO, Leff SE.
Journal: Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):14083-8.
PMID: 9391156 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers injected of either an AAV GDNF virus or an AAV-control (empty) virus into the substantia nigra region of the rat brain 3 weeks prior to delivery of a neurotoxin (6-OHDA). When they anlysed the brains 4 weeks after the neurotoxin was administered, they found that the AAV GDNF virus protected the dopamine neurons significantly better than the AAV-control virus. The AAV GDNF virus treated animals had 94% of their dopamine neurons intact, compared to just 51% in the AAV-control virus treated animals.
All of these successful results of the GDNF-based gene therapy in models of Parkinson’s led researchers to test this approach in non-human primates (with the goal of ultimately testing it in humans). Despite the ethical issues surrounding the use of primates in research, health regulators still require new treatments to be tested in them before they will give the green light for clinical testing in humans.
The first demonstration of GDNF-based gene therapy in primates involved the delivery of a lentivirus containing GDNF DNA into a primate model of Parkinson’s. The results of that study were published in 2000:
 Title: Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease.
Title: Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease.
Authors: Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Déglon N, Aebischer P.
Journal: Science. 2000 Oct 27;290(5492):767-73.
PMID: 11052933
In this study, the researchers found that lentivirus-GDNF reversed the behavioural/motor deficits in a neurotoxin (MPTP)-based primate model of Parkinson’s and completely prevented dopamine neuron degeneration. And this lentivirus result has been replicated (Click here to read more about this).
But researchers have chosen to use AAV viruses in the clinical testing of GDNF-based gene therapy and this has also been tested in primates, with very positive results:
 Title: Recombinant adeno-associated viral vector (rAAV) delivery of GDNF provides protection against 6-OHDA lesion in the common marmoset monkey
Title: Recombinant adeno-associated viral vector (rAAV) delivery of GDNF provides protection against 6-OHDA lesion in the common marmoset monkey
Authors: Eslamboli A, Cummings RM, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Kirik D, Annett LE.
Journal: Exp Neurol. 2003 Nov;184(1):536-48.
PMID: 14637123
And all of these results collectively gave the research community confidence in taking GDNF gene therapy to clinical testing for Parkinson’s.
Hang on a second, these reports were published in 2000 and 2003. Why has the clinical trial taken so long?
Because at the time that these reports were being published, GDNF was being clinically tested in Parkinson’s using a direct administration of GDNF protein approach (tubes were inserted into the brains of participants and GDNF was periodically infused), which…. well, let’s just say it has been a bit of a roller coaster saga (Click here to read more about that).
I see. So what did the new clinical study involve?
This Phase I clinical trial, involved 13 people with advanced Parkinson’s having a one-time injection of AAV2 virus containing GDNF-DNA injected into a region of the brain called the putamen on both sides of the head. The goal of the study was to investigate the safety, tolerability, and potential clinical effects of this treatment. There were three different doses of GDNF AAV virus used in this study (a low dose (9 x 1010vg in 6 participants), a medium dose (3 x 1011vg in 6 participants); and a high dose (9 x 1011vg in 1 participant)) (Click here to read more about the details of this trial – though please note that some of the trial details have changed).
One quick question: what is the putamen?
As I mentioned above, dopamine neurons in the brain reside in an area called the substantia nigra, near the base of the brain, but they project their branches (or axons) to the several other areas, including the putamen, and this is where they release most of their dopamine.
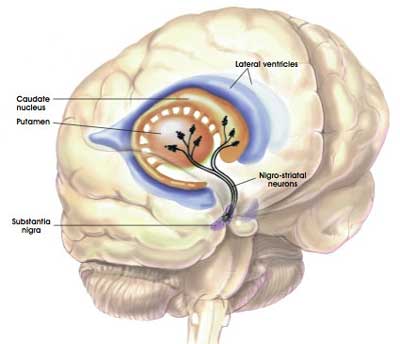 The projections of the substantia nigra dopamine neurons & location of the putamen. Source: MyBrainNotes
The projections of the substantia nigra dopamine neurons & location of the putamen. Source: MyBrainNotes
Appreciating that the putamen is where many of the axons of the dopamine neurons can be found, the researchers hoped that by delivering GDNF to that region they would encourage the dopamine neurons to not only survive, but also to grow more branches. An example of regenerative medicine.
Previous research in models of Parkinson’s suggested that delivering GDNF to the substantia nigra protected the dopamine neurons, but not their branches. By delivering GDNF to the putamen, the researchers may be able to protect both the cell bodies and the branches.
Let’s continue: Both pre-operatively and at 6-12 month intervals post-operatively, the participants in the study were assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) to determine any benefits in their motor function. They were also given brain imaging (PET), which was conducted to measure any change in dopamine activity.
And what did the results suggest?
So all I know is what has been provided in the abstract, but it sounds like the participants tolerated the AAV2-GDNF treatment very well.
In addition, the UPDRS clinical assessment scores remained stable across the time frame of the study, and in 12 of the 13 participants there was a 54% increase in dopamine activity at 18 months after AAV2-GDNF treatment (this effect ranged between 8-130% across the paritipants).
Based on these satisfactory results, the researchers are now planning a follow-up clinical trial (Click here to read the abstract).
Do we know anything about the follow up study?
The Phase I study was coordinated by Prof Krystof Bankiewicz of University of California, San Francisco (UCSF).
 Prof Bankiewicz. Source: Swiatlekarza
Prof Bankiewicz. Source: Swiatlekarza
And this summer (2019), he will be setting up a Phase II clinical trial for AAV-GDNF with the help of a private-public entity, called Brain Neu Bio. The sites for the larger Phase II will include those that were in place for Phase I study at UCSF and new sites at Ohio State University and in Europe (specifically Warsaw, Poland).
The new phase II trial will differ from the Phase I study in that they will be:
- Enrolling people with moderate Parkinson’s instead of moderate-to-severe PD
- Delivering the virus from the back of the skull, rather than the top of the skull
- Increasing the vector volume (the volume is expected to triple in an effort to increase the coverage of the putamen to at least 50% (it was only 26% coverage in the Phase I study)
By increasing the coverage of the putamen (that is, infecting more cells in the putamen with the AAV-GDNF virus treatment), the researchers are hoping to produce more GDNF. Ideally, more GDNF will produce a better outcome.
There is currently no details regarding the size of the study or format, but we will keep an eye out for them and mention them here when they become available (Click here to read more about what is known regarding this proposed Phase II study).
So what does it all mean?
GDNF is a contenscious topic for many folks in the Parkinson’s community – it represents hope to some, and gets the blood boiling in others. And there is certainly going to be more discussion about it as the Bristol GDNF clinical trial results get dissected, digested, and absorbed (Click here for a great over view of the current discussions that are being had on this topic). As such, I was almost reluctant to write this post for fear of the consequences (raised expectations, etc). But better sense won the day.
As we wait for the results of the Herantis Pharma clinical trial of CDNF (another neurotrophic protein) in Parkinson’s, it is encouraging for the neurotrophic field that this new GDNF clinical trial is being reported. Neurotrophic proteins represent one method of regenerative medicine for Parkinson’s. It is important to remember, though, that this current study is very small (only 13 individuals), it is ‘open-label’ (which means that everyone involved knows what is happening and what treatment is being administered), and it was not designed to test the efficacy of the GDNF AAV treatment. Thus, we must be very careful with our interpretation of any results.
I will be looking out for the publication of the full results of this study and for any news of a larger follow-up study.
The banner for today’s post was sourced from

Simon,
Thanks for the update on GDNF.
At the risk of turning your blog into “The Statistics of PD”, I’d like to focus on the data provided in the Abstract of the paper that you reference. I realize that it was a Phase 1 trial, but I’d like to squeeze as much out of the data as possible.
UPDRS Part 3 remained stable. Although these scores would, by the nature of a progressive disease, probably have worsened in the case of non-intervention, this is clearly different from the Bristol trial, where even the patients on placebo showed on average improved scores.
“Increased [18F]DOPA uptake in the infused areas was seen bilaterally in 10/13 patients at 6 months (range: 5-274%, median: 36%), and in 12/13 patients at 18 months after infusion (range: 8-130%, median: 54%).” 10/13 patients showed increased FDOPA uptake at 6 months, what of the other three? What happened to the maximum scorer after 6 months in the following 12 months? His/her score must have decreased by at least a third between 6 and 18 months (and more if he wasn’t the top scorer in this later period).
If I have a point to make, it is that before any future trials we need to be confident of the metrics that we use.
LikeLiked by 1 person
Hi John,
Thanks for your comment, but I’m not taking the bait. Given the lack of information in this abstract, I am inclined to accept that the treatment was safe & well tolerated, but I don’t want to speculate any further than that. It is particularly difficult to judge these results as 3 different doses were used and yet the outcomes are collectively provided. We’ll have to wait till the results are published (I understand that a manuscript is in production) before saying much more.
Kind regards,
Simon
LikeLike
Placebo effect is often more pronounced when surgery is involved in the treatment, which might (possibly) explain why the trial involving more extensive surgery to implant a permanently-present surgical apparatus had an enhanced placebo effect.
LikeLike
Hi Lou,
This is a very real possibility.
Kind regards,
Simon
LikeLike
Since neuroinflammation is usually a significant factor in PD, it seems to me that the variation in results (and the overall “not significant vs. placebo” finding with respect to UPDRS) of the *Bristol* GDNF trial *might* be related to the inflammatory effects of the extensive surgical procedures required.
This AAV approach to enhancing GDNF would seem to involve much less surgical intervention and its consequent trauma.
Do you think that *perhaps* the AAV-based procedure had less of an aggravating effect upon inflammation, allowing the positive effects of the GDNF to be perceived without being masked by enhanced inflammatory effects of the procedure?
I’m imagining that some patients in the Bristol trial may have experienced more inflammation from their surgeries than others. I do remember that one patient had such a poor response that they did his surgery over again, with improved results, which suggests (to me) that there might be a surgical factor in the variation between patients.
Since Bristol was a fairly small study, such random variations might have been significant, and that suggests to me that we should be looking at those patients in the GDNF group who improved very dramatically – a level of improvement that was not seen in *any* of the non-GDNF patients, as pointed out in your article on the Bristol trial.
So, the *impression* I’m getting is that providing GDNF to the putamen is indeed helpful, all other things being equal (e.g., without varying amounts of surgical trauma). And also that using the AAV as a delivery system for GDNF, by involving less trauma, removed some of the extraneous factors of the more severe surgical intervention, and allowed the “no change” in UPDRS values where some decline would normally be expected.
My reading of your report is that there was no placebo control group in the AAV/GDNF study, so I suppose the implied control would be the general expectation that there would have been some UPDRS decline over the study interval *without* the AAV/GDNF treatment.
The breakdown by dosing level of the AAV study will certainly be of interest once the full study has been published.
I have to say that I find this report *very* hopeful, despite your best efforts to control expectations :-).
One more comment:
It is interesting to see that once again, just as in the Bristol study, dopaminergic activity is substantially increased, but without any increase in UPDRS. Does that suggest that there are other (perhaps complementary) parts of the brain involved in motor activity, which have declined in tandem with the loss of dopamine neurons, and which continue to limit motor function despite the improvement in dopaminergic processing?
Great report; very grateful for your coverage of these issues.
LikeLike
Sorry, in the above comment where I refer to a decline in UPDRS I meant to say a decline in *function* as *indicated* by UPDRS (i.e., an increase in UPDRS). And where I refer to an increase in UPDRS I meant to say an increase in *function* as *indicated* by UPDRS.
LikeLike
Hi Lou,
Thanks for your interesting comment – I hope all is well.
The AAV-GDNF approach is still brain surgery, but less invasive than the Bristol GDNF procedure – which left treatment delivery tubes in the brain. The AAV-GDNF approach is a one time delivery of virus on both sides of the brain. There will definitely be some post surgical inflammation, but this will most likely reside with recovery.
At present, all I am prepared to say is that providing GDNF to the putamen appears to be safe and well tolerated. Whether it is beneficial is still to be determined (a controversial & potentially inflammatory statement! But sitting on the fence is the safest place for me). One question I am always asking in the translation from preclinical models to the clinic is whether the human brain is static in response to PD. My feeling is that it isn’t, changing and adapting over time as the disease progresses and its circumstances change. Does this render it less responsive to GDNF (or other preclinical miracles) over time? Animal models are rather static in comparison.
One potential cautionary point to the continuous delivery of GDNF (which would be the case in this AAV-GDNF situation – infected cells will simply continue to produce GDNF) is that this may not be ideal for regenerative medicine. There has been some recent research suggesting that an intermittent approach is more ideal, rather than a continuous flood of GDNF (see this report for more on this: https://www.ncbi.nlm.nih.gov/pubmed/30649249 ). Such research is more supportive of the Bristol GDNF approach than the AAV-GDNF treatment. This should not stop the proposed Phase II AAV-GDNF study, as GDNF delivery to the putamen may well protect/rejuvenate remaining dopamine neurons and their fibres, but it is something to keep in mind.
I too am intrigued by the disconnect between the brain imaging data and the clinical outcome. I think the same thing was also seen in the AAV-Neuturin clinical trials, but I can’t currently find the results. Perhaps this reflects changes elsewhere in the brain which counter-act improvements in the putamen. This harkens back to my comment above regarding changes and adaption over time in the brain in response to PD. Perhaps with longer duration studies, the brain will again adapt and clinical benefits will become evident. This is just me speculating and needs to be tested.
Kind regards,
Simon
LikeLiked by 1 person
Any chance GDNF and other neutropics are enhancing system dopamine ‘consumption’ in parallel to increasing production for a net zero effect? Higher actively but Few newly lit or improved circuits?
It would be interesting to see the relative DAT to udprs ratios for voyager/stem cell approaches.
LikeLiked by 1 person
Hi Double,
That’s a REALLY interesting idea. But a slight clarification before delving any deeper: DATScans only tell us how much dopamine transporter protein there is in the brain. It doesn’t actually tell us if there is more dopamine. Nor does it tell us if there is more dopamine neuron fibres. These last two concepts are simply speculations based on DATscan imaging results. And I have to admit that I am guilty of over simplifying DATscans in many of the SoPD post (by describing it as “a measure of dopamine activity in the brain”), if only to keep the posts from going on forever.
I really do like the idea of comparing DATScan/clinical outcome results between all of these clinical studies though. I will reach out to some of those involved and make some inquiries. Thanks for the genius idea – will let you know the outcome of the correspondence.
Kind regards,
Simon
LikeLiked by 1 person
I can certainly understand your desire to avoid the inflammatory effects of premature speculation; preventing inflammation is essential in all matters pertaining to PD.
But in my own position, there’s really no down side, as I’m not in a position where people would care very much about what I think or post online.
So, then, allow me to add one additional card to my house, and look out for falling cards below.
Here is the concept: What if GDNF is causing an increase in axonal branching (as I have read that it might do), resulting in additional dopamine axon terminals in the putamen? And what if these new axon terminals produce additional dopamine and additional firings, but are not usefully engaged with synaptic terminals, so that the dopamine they produce goes out into inter-cellular space and then just gets recycled into those same (or other) neurons via the dopamine transporter, so that the additional terminals are in effect “spinning their wheels?”
If that were the case, then indeed perhaps a longer-term trial would give these new terminals time to hook up to synaptic terminals and have some effect upon UPDRS.
Or, perhaps that might never occur, regardless of the time allowed.
In either case, it might explain the discrepancy between the increased DAT activity and the lack of significant changes to the UPDRS.
I’m not familiar with the details of synaptogenesis, so I’m not sure whether it is even possible for a newly-created axon branch to have a terminal that is in some incomplete stage of synaptogenesis where it is producing dopamine into the synaptic cleft which is not triggering anything on the receiving side of that synapse. It’s just a general idea.
LikeLike
Thank you, Simon. So far so good. One critical question: were the dopamine uptake improvements dose dependent?
LikeLike
Hi Tom,
Thanks for your question. The abstract does not provide that kind of detail, so we’ll have to wait till the results are published.
Kind regards,
Simon
LikeLike
Thanks as always Simon!
LikeLike