|
Researchers at the University of Cambridge have published an interesting research report last week regarding a clinically available drug that they suggest boosts autophagy in the brain. Autophagy is one of several processes that cells use to dispose of waste and old proteins. The drug is called Felodipine, and it is a calcium channel blocker that is used to treat high blood pressure. In today’s post, we will look at what autophagy is, how boosting it could help with neurodegenerative conditions, and whether Felodipine should be clinically tested for re-purposing to Parkinson’s.
|
 Source: Novusbio
Source: Novusbio
This is Prof David Rubinsztein (blue shirt) and the members of his research lab at the Cambridge Institute for Medical Research (CIMR) in Cambridge (UK).
 Prof Rubinsztein is the Deputy Director of the CIMR, the Academic Lead of the UK Alzheimer’s Research UK Cambridge Drug Discovery Institute, and he is a group leader at the UK Dementia Research Institute at the University of Cambridge.
Prof Rubinsztein is the Deputy Director of the CIMR, the Academic Lead of the UK Alzheimer’s Research UK Cambridge Drug Discovery Institute, and he is a group leader at the UK Dementia Research Institute at the University of Cambridge.
He is also one of the world’s leading experts in the field of autophagy in neurodegenerative conditions.
What is autophagy?
Autophagy (from the Ancient Greek αὐτόφαγος autóphagos, meaning “self-devouring”) is an absolutely essential function in a cell. Without autophagy, old proteins would pile up, making the cell sick and eventually causing it to die. Through the process of autophagy, the cell can break down the old protein, clearing the way for fresh new proteins to do their job.

The process of autophagy. Source: Wormbook
Waste material inside a cell is collected in membranes that form sacs (called vesicles). These vesicles then bind to another sac (called a lysosome) which contains enzymes that will breakdown and degrade the waste material – the same way enzymes in your washing powder break down muck on your dirty clothes). The degraded waste material can then be recycled or disposed of by spitting it out of the cell.
Here is a video of Prof Rubinsztein explaining autophagy:
Why are researchers interested in autophagy in neurodegeneration?
Most of the major neurodegenerative conditions (Alzheimer’s, Parkinson’s, Huntington’s, etc) are associated with the accumulation of aggregated proteins. Autophagy is considered one cellular process that could be therapeutically targetted to help clear the brain of aggregated proteins.
What are aggregated proteins?
These are particular proteins that accumulate and then clump/stick together in these conditions. Many of these proteins are ‘misfolded’ (meaning that they are not in their correct shape/confirmation). Some of the proteins are similar across conditions, but when we think of Parkinson’s the primary aggregated protein that we discuss is alpha synuclein.
What is alpha synuclein?
Alpha synuclein sounds like a distant galaxy, but it is one of the most common proteins in our brains. It makes up about 1% of all the protein in a neuron. When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure. When it is first produced, alpha synuclein will look something like this:
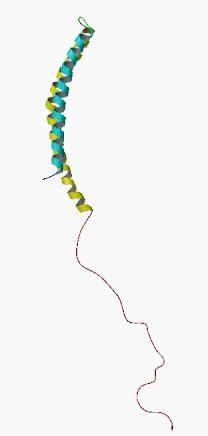
Alpha synuclein. Source: Wikipedia
In this form, alpha synuclein is considered a monomer – which is a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). It is believed that alpha synuclein has certain functions as a monomer, but may also have specific tasks as a oligomer.
In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.

Microscopic images of Monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein that aggregate together, and then go on to form what we call Lewy bodies.
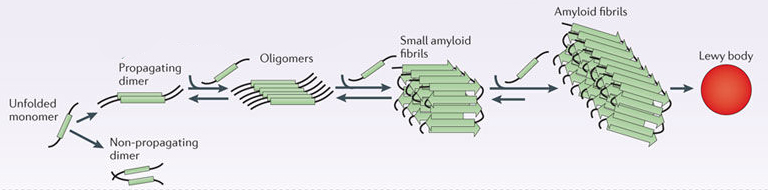 Parkinson’s associated alpha synuclein. Source: Nature
Parkinson’s associated alpha synuclein. Source: Nature
What are Lewy bodies?
Lewy bodies are dense circular clusters of aggregated alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
Lewy bodies are one of the cardinal features of the Parkinsonian brain – they are used to help make postmortem diagnoses.
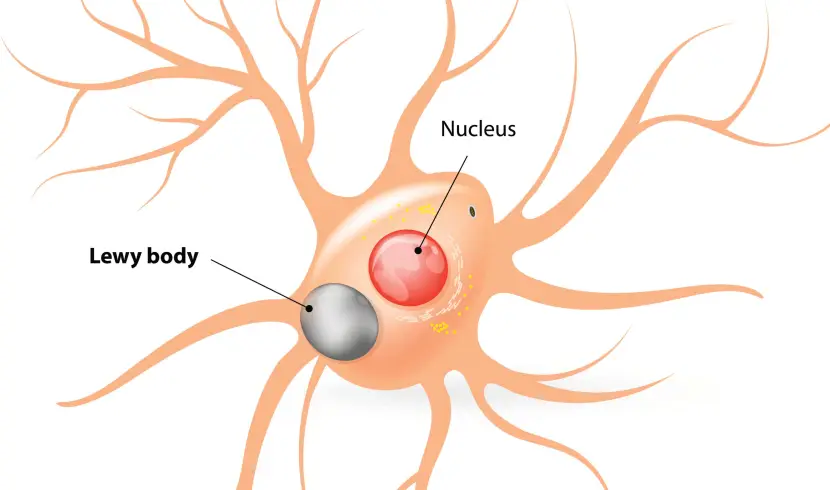 A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
The aggregated alpha synuclein protein, however, is not limited to just the Lewy bodies.
In the affected areas of the Parkinsonian brain, aggregated alpha synuclein can be seen in the branches (or neurites; see black arrow in the image below) of cells. In the image below, alpha synuclein has been stained brown on a section of brain from a person with Parkinson’s.

Examples of Lewy neurites (stained in brown; indicated by arrows) from a human brain. Source: Wikimedia
By increasing autophagy in the brain, researchers hope to be able to reduce the amount of aggregated protein in cells. This action will hopefully reduce the stress on cells caused by aggregated protein and also make them more healthy and help them to function better. And this is why researchers are interested in autophagy in neurodegenerative conditions.
In Parkinson’s, there is a great deal of research effort focused on increasing autophagy to clear aggregated alpha synuclein from affected cells (Click here to read a recent SoPD post about a new clinical trial in New Zealand exploring this idea).
Ok, I see. So what have Prof Rubinsztein and his team recently discovered?
They recently published this report here:
 Title: Felodipine induces autophagy in mouse brains with pharmacokinetics amenable to repurposing
Title: Felodipine induces autophagy in mouse brains with pharmacokinetics amenable to repurposing
Authors: Siddiqi FH, Menzies FM, Lopez A, Stamatakou E, Karabiyik C, Ureshino R, Ricketts T, Jimenez-Sanchez M, Esteban MA, Lai L, Tortorella MD, Luo Z, Liu H, Metzakopian E, Fernandes HJR, Bassett A, Karran E, Miller BL, Fleming A, Rubinsztein DC
Journal: Nat Commun. 2019 Apr 18;10(1):1817.
PMID: 31000720 (This report is OPEN ACCESS if you would like to read it)
In this study, Prof Rubinsztein and his team evaluated a class of drugs that they had identified as autophagy activators from a previous study.
This was the previous study:
 Title: Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway.
Title: Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway.
Authors: Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, O’Kane CJ, Floto RA, Rubinsztein DC.
Journal: Nat Chem Biol. 2008 May;4(5):295-305
PMID: 18391949 (This article is OPEN ACCESS if you would like to read it)
In this previous study, the researchers screened 253 FDA-approved drugs to identify any that displayed autophagy-activating properties.
They were using models of Huntington’s disease to identify these drugs.
What is Huntington’s disease?
Huntington’s disease is a neurodegenerative condition which results in individuals losing all inhibition of movement, giving rise to appearance of chorea – jerky, random, and uncontrollable movements (similar to dykinesias).
Huntington’s disease is a genetic condition, caused by an increase in a region of DNA inside the Huntingtin (Htt) gene.
This regions is made up of CAG repeats. In normal healthy humans, we usually have up to 30 repeats of CAG. If you have more than 40 CAG repeat, you are definitely going to develop Huntington’s disease. It is a simple diagnostic test.

Expansion of CAGs in the Huntingtin gene. Source: NIST
The expansion of the CAGs in the Huntingtin gene results in a mutant form of the Huntingtin protein. This mutant Huntingtin protein clusters and forms aggregates, and is believed to be associated with the cell death and clinical features observed in people suffering from Huntinton’s disease.
Prof Rubinsztein and his team are seeking to reduce the aggregates in the brains of people with Huntington’s disease by increasing autophagy. Hence their search for autophagy activators. And by screening clinically available, FDA-approved drugs, they are hoping to speed up the process of identifying such activators and get them to the patients quicker. And this may have beneficial effects for more than just the Huntington’s community.
In their previous screening study, the investigators identified three clinically available activators of autophagy:
- The L-type Calcium channel blocker, Verapamil
- The K+ATP channel opener, Minoxidil
- The Gi signaling activator, Clonidine
All of these drugs shared similar biological properties which the researchers investigators explored in that first report. But in their more recent follow up study, the researchers have focused on L-type Calcium channel blockers.
What is a L-type Calcium channel blocker?
L-type Calcium channel blockers are a class of drug which are used for the treatment of cardiac antiarrhythmia ( abnormal rhythms of the heart) or hypertension (high blood pressure).
And what did the researchers report in their new study?
Firstly, they compared five different L-type Calcium channel blockers to see if any of them displayed better autophagy activating properties to that seen in Verapamil.
And they found that one of them did: Felodipine
The researchers found that treating cells with Felodipine (also known as Plendil) increased the number of autophagic vesicles in cells more than Verapamil.
 Felodipine increased autophagy more than Verapamil. Source: Nature
Felodipine increased autophagy more than Verapamil. Source: Nature
They next tested Felodipine in several different models of neurodegeneration involving protein aggregation and found it was very good at reducing levels of aggregated protein in each of the models (Huntington’s and dementia). They also tested the drug in an alpha synuclein-based model of Parkinson’s.
Specifically, the researchers used A53T mice.
Source: Pinterest
What are A53T mice?
The section of DNA that gives rise to alpha syncuclein protein is called SNCA. And there are several genetic variations inside of SNCA that are associated with an increased risk of developing Parkinson’s. A53T is the name of one of those genetic variations.
As you can see in the image below, A53T lies in the red (Amphipathic) region of SNCA along with several other genetic variants, such as A30P and E46K:
Mice have been genetically engineered to carry the human SNCA gene with the A53T genetic mutation (Click here to read the original report). These mice initially exhibit hyperactivity and then start to display signs of alpha synuclein protein accumulation and aggregation at about four to six months of age. They also pass away earlier than normal mice (12-14 months of age, compared to 20+ months for normal mice).
The researchers found that treating mice carrying this A53T genetic variation with Felodipine for 28 days (starting at 6.5 months) significantly reduced the amount of alpha synuclein protein accumulating in the brains of these mice.
 Less alpha synuclein accumulation. Source: Nature
Less alpha synuclein accumulation. Source: Nature
Felodipine treatment also improved the behavioural deficits observed in these mice and reduced the number of dopamine neurons being lost. And importantly, these results were achieved at doses of Felodipine which are equivalent to those used in humans.
 Better motor ability and more dopamine neurons. Source: Nature
Better motor ability and more dopamine neurons. Source: Nature
The researchers concluded that their “data suggest that felodipine induces autophagy in neurons and enhances removal of a range of disease-causing proteins: mutant huntingtin, mutant α-synuclein and tau“. They acknowledge that they “are not aware of any data on felodipine brain concentrations in humans“, which would need to be evaluated before any clinical trialling of this drug for re-purposing for neurodegenerative conditions.
The results of this study are interesting, but
Has anyone ever clinically tested other calcium channel blockers in Parkinson’s?
So this is a very delicate question to answer.
As is the actual timing of this new Felodipine study.
You see, there is a clinical trial that has been testing a calcium channel blocker in Parkinson’s, and the results of that study are just about to be announced. I am reluctant to mention this as I do not want to raise hopes or expectations, in case the results are not agreeable. But given that the results are imminent, I am mentioning it and we will tread very carefully.
On the 4th May, the results of the STEADY-PD III study will be announced at the annual meeting of the American Academy of Neurology in Philadelphia.
 Source: STEADY-PD III
Source: STEADY-PD III
STEADY-PD III is a Phase III clinical trial that is investigating the utility of the calcium channel blocker ‘Isradipine’ (tradenames DynaCirc, Prescal) for Parkinson’s.
 Isradipine. Source: Dailymed
Isradipine. Source: Dailymed
Isradipine is a calcium channel blocker of the dihydropyridine class (similar to Felodipine). Prof Frank Church over at the ‘Journey with Parkinson’s’ blog has a great page on isradipine (Click here to read that post).
The double blind, Phase III STEADY-PD clinical trial has been conducted at 56 research centres across the US & Canada, enrolling over 300 people with newly diagnosed Parkinson’s to take either isradipine or a placebo treatment for 36 months (participants are randomly assigned to one of the two groups – click here to read more about the clinical study).
We wait to see what the results suggest.
Have any associations between L-type calcium channels and Parkinson’s been reported?
About nine years ago, this report was published:
 Title: L-type calcium channel blockers and Parkinson disease in Denmark.
Title: L-type calcium channel blockers and Parkinson disease in Denmark.
Authors: Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S.
Journal: Ann Neurol. 2010 May;67(5):600-6.
PMID: 20437557 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers collected the medical records of 1,931 people in Denmark who were diagnosed with Parkinson’s between 2001 and 2006. These records were age and sex matched to 9,651 records of healthy controls from the same register. After analysing all of the records, the investigators found that people prescribed with L-type calcium channel blockers (also known as dihydropyridines) were 27% less likely to develop Parkinson’s.
This finding supported a previous study that found a similar result (Click here to read more about that) and similar results have been independently reported (Click here and here to read those reports).
And this finding was also supported by postmortem analysis of the Parkinsonian brain:
 Title: Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins.
Title: Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins.
Authors: Hurley MJ, Brandon B, Gentleman SM, Dexter DT.
Journal: Brain. 2013 Jul;136(Pt 7):2077-97.
PMID: 23771339 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers looked at where in the human brain the L-type calcium channels were present and in what concentration. They used sections of postmortem brain to carry out this analysis, looking at both healthy specimens as well as those from people who passed away with Parkinson’s.
In the normal brains, the distribution of the L-type calcium channels and certain calcium-binding proteins was not associated with regions of the brain that are prone to the selective neurodegeneration that is seen in Parkinson’s. But the investigators observed a very different picture in the Parkinsonian brains.
Increased levels of L-type calcium channels and the calcium-binding proteins were found throughout the brains of people who passed away with Parkinson’s. Even in cases of early Parkinson’s (in people who passed away shortly after being diagnosed), increased levels of L-type calcium channels were even found in the cerebral cortex – a part of the brain largely unaffected in Parkinson’s.
These findings lead the researchers to conclude that “disturbed calcium homeostasis” may be “an early feature of Parkinson’s disease and not just a compensatory consequence to the neurodegenerative process”.
So yes, we can say that there is data suggesting some associations between L-type calcium channels and Parkinson’s.
But there is also some conflicting data:
 Title: Dihydropyridine calcium channel blockers and the progression of parkinsonism
Title: Dihydropyridine calcium channel blockers and the progression of parkinsonism
Authors: Marras C, Gruneir A, Rochon P, Wang X, Anderson G, Brotchie J, Bell CM, Fox S, Austin PC.
Journal: Ann Neurol. 2012 Mar;71(3):362-9. doi: 10.1002/ana.22616.
PMID: 22451203
Using data were obtained from Ontario’s health care administrative databases, the researchers analysed the records of 4,733 hypertensive individuals with parkinsonisms. Specifically, they were assessing if there was any delay in the time taken to initiate drug treatment for parkinsonisms, nursing home admission, or death.
The investigators found that longer term treatment with any dihydropyridine was associated with a decreased risk of each of the three outcomes. But they found no evidence of a difference in effect between the brain-penetrant dihydropyridines compared with dihydropyridines with limited ability for brain penetration, which the researchers suggested does not support the idea of this class of drugs is having a meaningful effect on the progression of parkinsonism
Thus, we must be careful in how we proceed with interpreting the new results.
And we have been to this rodeo before – in the case of the SURE-PD Inosine clinical trial for Parkinson’s.
On the 10th December 2018, the National Institute of Health recommended the early closure of the Phase III clinical study of Inosine in Parkinson’s, due to failure to achieve the primary end point of the study (demonstrating efficacy in slowing disease progression – Click here to read the press release about this).
This was despite the fact that all of the initial preclinical data was very supportive of clinical evaluation of inosine and there were good association in previous clinical data which improved the case for support (there will be a SoPD post on inosine once the Phase III results are published).
 While the new Felodipine results are interesting for neurodegenerative conditions in general, it is probably wise to wait for the STEADY-PD III clinical trial results before we start speculating on next steps for Felodipine in the context of Parkinson’s.
While the new Felodipine results are interesting for neurodegenerative conditions in general, it is probably wise to wait for the STEADY-PD III clinical trial results before we start speculating on next steps for Felodipine in the context of Parkinson’s.
So what does it all mean?
Researchers at the University of Cambridge have identified a clinically available drug that boosts the waste disposal system of cells, suggesting that this treatment could be re-purposed for neurodegenerative conditions associated with an accumulation of old proteins. The drug is a calcium channel blocker called Felodipine and it is used to treat blood pressure.
 Source: Medicinehow
Source: Medicinehow
While these new results are very encouraging, it will be important to see independent replication of these findings and the results of a Phase III clinical trial in Parkinson’s which has tested a very similar drug for its ability to slow the progression of the condition, before folks start to get excited though.
As always, cautious optimism is required.
Or as Parkinson’s advocate Martin Taylor puts it ‘Positive realism‘ .
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Netdoctor



SIMON ITS STEVE LOPEZ .. FORMER COP, NOW RETIRED ER DOC FROM BUFFALO, NY USA CHECKING IN … I WOULD HOPE YOU CONSIDER LETTING YOUR READERS KNOW THAT IF THEY DESIRE TO PARTICIPATE IN A STUDY/TRIAL AND ARE CONSIDERING DBS.THEY SHOULD THINK TWICE. I HAVE LITERALLY BEEN REJECTED FROM EVERY FREAKIN STUDY I HAVE CONTACTED DUE TO MY HAVING DBS .. That just sucks … cancer patients have more hope to get into disease-modifying †rials/studies than those of us who have undergone DBS… MAKES ME FEEL LIKE I AM JUST WAITING TO DIE NOW …. The information in this communication, including all attachments transmitted with it, is confidential and may be legally privileged. It is intended solely for the addressee. No confidentiality or privilege is waived or lost by any transmission. If you are not the intended recipient, you are strictly prohibited from disclosing, copying, distributing or using any of this information. If you received this message in error, please contact the sender immediately and destroy the material in its entirety, whether electronic or hard copy. The sender does not accept liability for any errors or omissions. This communication may contain nonpublic personal information about consumers, subject to the restrictions of the Gramm-Leach-Bliley Act. You may not directly or indirectly reuse or disclose such information for any purpose other than to provide the services for which you are receiving the information.
LikeLike
Steve, I am very sorry to hear that you have been excluded from clinical trials because of your previous treatment.
I’m not suffering from PD, only had suspicions that I was had RBD, and my father had PD in his late 70’s. So I started studying possible PD and anti-aging protocols. Because I was already taking a CCB, I switched to Isradipine from amlodipine. I also take Metformin, a low dose of Selegiline (5mg) everyday based on the research conducted by its discoverer for longevity. I also am using Senolytics (so far Fisetin, Quercetin and Azithromycin, will start on Dasatinib soon), and then will start using Rapamycin on a low-dose weekly basis. I also take daily low-dose Aspirin, Tadalafil and Telmisartan, and 2 grams of Vitamin B3 with 10mg of Atorvastatin because each of these drugs have been shown to be effective in anti-aging regimens.
I have a low-carb diet and am in better shape at 65 than I was 20 years ago. I can drop and give you 50, a hundred Sit-Ups and lift weights three times a week.
I guess that what I am saying is that you don’t need to wait on Clinical studies. Nothing that they are testing appears to be unavailable to the common man or woman. I conduct my OWN trials with things shown not to be deleterious to my health, and I am doing well, so far.
You were a cop. Don’t let the bad guys (i.e., the conditions you find yourself in) get the better of you!
Best of luck!
LikeLike
https://memory.ucsf.edu/research-clinical-trials
https://clinicaltrials.ucsf.edu/
https://clinicaltrials.ucsf.edu/browse/
LikeLike
So, I presume that impaired autophagy is not considered a ‘cause’ in most cases of neurological disease. Otherwise, wouldn’t increased buildup of aSyn as well as bAmyloid and others simultaneously result, giving the patient not just Parkinson’s but also Alzheimer’s, Huntinton’s, CZ and who knows how many other conditions at the same time? Best hope is that it can at least partially remove the accumulation of cellular rubbish, slowing disease progression.
LikeLike
In more general terms, isn’t autophagy caused/helped by fasting for a period of time (some say 24/48 hours)?
And if this is so, would fasting slow or stop the build up of accumulation of aggregated proteins and so slow the progression of Parkinsons itself?
LikeLiked by 1 person
The Bredesen Protocol for AD incorporates the time-restricted eating version of fasting (just 12 hours nightly) – along with a number of other interventions.
LikeLike
Great post Simon! Fingers crossed for steady pd results. Three years is a long time to be on a placebo or a drug that doesn’t work. Hopefully we’ll get some good news.
LikeLike
obviously, nobody is waiting to declare major failure exactly on the annual meeting of the American Academy of Neurology
LikeLike
And yet that’s just what they did.
LikeLike
Is this the end of plendil as well?
LikeLike