|
Earlier this year, a San Francisco-based biotech company – called Cortexyme – published a research report that grabbed my attention. The study presented data supporting an alternative theory of the cause of Alzheimer’s – one in which a bacteria involved in gum disease appears to be playing a leading role – and evidence that the company’s lead experimental compound COR388 could have beneficial effects in the treatment of the condition. While the study was intriguing, what completely blew my mind was the fact that the company had already tested COR388 in a couple of Phase I clinical trials, and since then they have initiated a large Phase II/III trial. In today’s post, we will discuss this new theory of Alzheimer’s, look at what Cortexyme are doing, and how this could relate to Parkinson’s.
|
The dashed lines show associations. Source: Slideplayer
Before we start today’s post, a word on ‘associations‘.
Please remember while reading this material that association does not equate to causation.
So if I write something like “researchers have found an association between a type of bacteria that causes gum disease and Alzheimer’s”, it does not mean that someone with either condition necessarily has the other. It only means that they have both simply appeared in the same individuals at a higher than chance rate.
All clear?
Yes.
Good.
So what is today’s post about?
A very interesting report in which researchers have found an association between a type of bacteria that causes gum disease and Alzheimer’s.
Ok, remind me again – what is Alzheimer’s?
Alzheimer’s is the most common neurodegenerative disease, accounting for 60% to 70% of all cases of dementia. It is a progressive neurodegenerative condition affecting approximately 30 million people around the world.
The condition is characterised by a global loss of cells in the brain.
 The brain on the right had Alzheimer’s. Source: BrainRepair
The brain on the right had Alzheimer’s. Source: BrainRepair
Inside the brain, in addition to the cell loss, there are two cardinal features of the Alzheimer’s brain:
- Neurofibrillary tangles
- Amyloid plaques
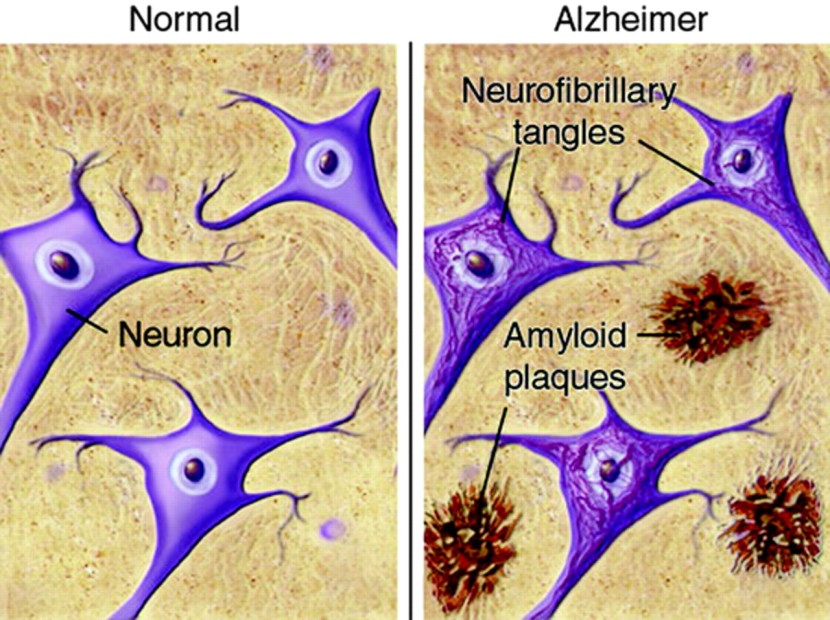
A schematic demonstrating the difference between healthy and Alzheimer’s affected brains. Source: MmcNeuro
The tangles are the clustering (or aggregation) of a protein called TAU (Click here to read a previous SoPD post on TAU). These tangles reside within neurons initially, but as the disease progresses the tangles can be found in the space between cells.
Amyloid plaques are clusters of protein that appear outside of the cells in Alzheimer’s. A key component of the plaque is a protein called beta amyloid.
Beta-amyloid has long been considered to be the villain in Alzheimer’s.
It is a piece of a larger protein that sits in the outer wall of nerve cells where it has certain functions. In certain circumstances, specific enzymes can cut it off and it floats away.
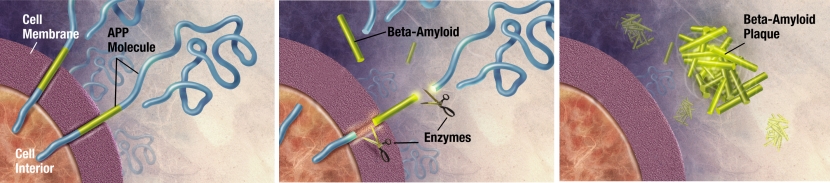
The releasing of Beta-Amyloid. Source: Wikimedia
Beta-amyloid is a very “sticky” protein and it has been believed that free floating beta-amyloid proteins begin sticking together, gradually building up into the large amyloid plaques. And these large plaques were considered to be involved in the neurodegenerative process of Alzheimer’s.
Thus, for a long time scientists have attempted to reduce the amount of free-floating beta-amyloid in the brain.
Unfortunately, clinical trials of treatments focused on removing beta-amyloid from the brain have failed to have any impact on the course of the condition (Click here to read more about this). And in some cases they may have made the situation worse (Click here to read more about this).
This situation has resulted in a major re-think of our understanding of Alzheimer’s.
And what are people thinking?
Well, some researchers have been asking whether beta amyloid is actually that bad.
Maybe the build up of this sticky protein is actually a good thing.
Que? What do you mean?
In 2016, this report was published:

Title: Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease.
Authors: Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, Moir RD.
Journal: Sci Transl Med. 2016 May 25;8(340):340ra72.
PMID: 27225182 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers wanted to explore what functions beta amyloid could be having in the brain. Specifically, they were interested in the idea that this sticky protein could be some kind of cellular defensive mechanism against infectious agents.
The investigators tested this idea by taking three types of mice:
- genetically normal mice
- mice with no beta amyloid
- mice which produce a lot of beta amyloid (called 5xFAD mice)
They then infected all of the mice with the microbe that causes meningitis, and waited to see what happened.
And what happened?
They found that the mice producing a lot of beta amyloid lived significantly longer than the other groups of mice.
 Source: PMC
Source: PMC
They next repeated the experiment in a species of microscopic worm – called C.elegans – and found similar results. These findings suggested that beta amyloid was having a positive effect in the brain – it was exhibiting anti-microbial properties which may represent some kind of defensive mechanism in the brain.
But then they noticed something curious.
Normally, the 5xFAD mice (which produce a lot of beta amyloid) do not develop a lot of protein aggregation until they reach old age, but when the researchers looked in the brains of the 5xFAD mice they had infected with meningitis, they found significant levels of aggregation in the mice producing a lot of beta amyloid but at a young age (and just 48 hours after infection).
This led the researchers to conduct some cell culture experiments in which they watched what was happening to the bacteria and beta amyloid. They found that the beta amyloid was sticking to the bacteria and this was leading to the formation of plaque-like protein aggregates.
The results of these experiments suggested to the researchers an intriguing possibility that beta amyloid may be playing a protective role in the brain – acting as an immune system for the brain – against infection.
 Source: Nature
Source: Nature
Thus, the aggregations we see in the brains of people with Alzheimer’s may not be the cause of the cell death associated with the disease, but rather evidence of the ‘brain’s immune system’ trying to fight back against an invisible and unknown infectious agents.
The researcher’s of the study were quick to point out that this antimicrobial action of beta amyloid is simply a novel function of the protein, and it may have nothing to do with the disease itself.
But it has been interesting watching where this research went next.
Where did it go next?
Well, firstly the researchers who conducted that study have expanded on their previous work by firstly better characterising the anti-microbial properties of beta amyloid protein (Click here to read that report), but also by presenting evidence that beta amyloid protein can bind to and prevent herpesvirus from infecting cells, supporting the notion that beta amyloid might have a protective role in brain immunity (Click here to read that follow up report).
 Herpes simplex virus. Source: Wikipedia
Herpes simplex virus. Source: Wikipedia
But more importantly, independent research groups have also observed anti-microbial/viral properties in beta amyloid protein (Click here, here, and here for some examples).
And then of course there was the report early this year, which is the focus of today’s post.
This report here:
 Title: Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors.
Title: Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors.
Authors: Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, Holsinger LJ, Arastu-Kapur S, Kaba S, Lee A, Ryder MI, Potempa B, Mydel P, Hellvard A, Adamowicz K, Hasturk H, Walker GD, Reynolds EC, Faull RLM, Curtis MA, Dragunow M, Potempa J.
Journal: Sci Adv. 2019 Jan 23;5(1):eaau3333
PMID: 30746447 (This is an OPEN ACCESS report if you would like to read it)
In this study, the researchers had noted that loss of teeth and periodontitis (or inflammation of the gums) is associated with dementia.
Really?!?
Really.
In 2016, this research report was published:
Title: Periodontitis and Cognitive Decline in Alzheimer’s Disease.
Authors: Ide M, Harris M, Stevens A, Sussams R, Hopkins V, Culliford D, Fuller J, Ibbett P, Raybould R, Thomas R, Puenter U, Teeling J, Perry VH, Holmes C.
Journal: PLoS One. 2016 Mar 10;11(3):e0151081.
PMID: 26963387 (This report is OPEN ACCESS if you would like to read it)
In this study, the investigators conducted a six month observational study on 60 people with mild to moderate Alzheimer’s. They were cognitively assessed and a blood sample taken at their first visit, and then again 6 months later.
The presence of periodontitis at baseline was not related to baseline cognitive state, BUT it was associated with a six fold increase in the rate of cognitive decline at the 6 month timepoint.
And a second research group found a similar association:
 Title: Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study
Title: Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study
Authors: Chen CK, Wu YT, Chang YC.
Journal: Alzheimers Res Ther. 2017 Aug 8;9(1):56.
PMID: 28784164 (This report is OPEN ACCESS if you would like to read it)
In this much larger study, the researchers used the Taiwan health system database to identify 9291 people with newly diagnosed with periodontitis (between 1997 and 2004). They matched them with the records of 18,672 people without periodontitis, and then further 10 year follow up analysis.
The results demonstrated that a 10-year period of periodontitis exposure was associated with a 1.7-fold increase in the risk of developing Alzheimer’s.
And there are lot’s of other examples of this association (Click here, here, here, here, and here for examples of research on this topic).
Whoa. I’m going to brush my teeth. And use mouth wash! So what happened next?
The investigators who conducted this more recent study (the focus of today’s post), questioned whether infection with Porphyromonas gingivalis could be involved with this association.
What is por…phy…ro…whatever?
Porphyromonas gingivalis is one of the key bacteria involved in the development of periodontitis.
 Porphyromonas gingivalis – cute huh? Source: Perioprosthocc
Porphyromonas gingivalis – cute huh? Source: Perioprosthocc
It causes an inflammatory disease in the gums of the jaw, which destroys the tissues supporting teeth, eventually leading to tooth loss. Porphyromonas gingivalis can invade gum cells (or gingiva cells) and can survive the presence of antibiotics.
It is a rather hardy little guy.
 Source: Researchgate
Source: Researchgate
So how did the researchers investigate this?
They firstly started by having a close look at tissue from postmortem brains from both people who passed away with Alzheimer’s and age-matched healthy controls.
And what they found was rather remarkable.
Bacterial proteins associated with porphyromonas gingivalis were significantly higher in the Alzheimer’s brain tissue samples (compared to the healthy control samples).
 RgpB & Kgp are two porphyromonas gingivalis proteins. Source: PMC
RgpB & Kgp are two porphyromonas gingivalis proteins. Source: PMC
They also found them in the cerebrospinal fluid – the solution that the brain sits in. This suggested to the researchers that this oral bacteria was somehow accessing the brain.
Next the researchers looked to see if gingipain are neurotoxic.
Wait a minute. What are gingipain?
Gingipain are proteases that is secreted by porphyromonas gingivalis.
And before you ask: proteases are enzymes which breaks down proteins. These gingipains are part of how porphyromonas gingivalis kills cells in the gums. Both RgpB & Kgp (mentioned in the image above) are gingipains.
The researchers injected mice with a combination of gingipains that porphyromonas gingivalis secrets, and they found that these mice had a significantly greater number of degenerating neurons in their brains (compared to saline-injected mice). The investigators also reported that this neurodegeneration could be blocked by pretreating the mice with a combination of gingipain inhibitors.
One interesting detail here: beta amyloid levels did not increase in the gingipain injected mice (we’ll come back to this in the next sentence).
 Source: ScienceLife
Source: ScienceLife
Next, the researchers orally infected mice with porphyromonas gingivalis every two days over a 6 week period and this resulted in a significant increase in levels of beta amyloid protein in the brain (compared to control treated mice). So the increase in beta amyloid protein levels was specific to the bacteria and not its secreted proteins.
Infecting mice with porphyromonas gingivalis also resulted in significant cell loss in analysed areas of the brain, but this cell loss was completely prevented by daily treatment with gingipain inhibitors (starting at day 36, until day 70 when the brains were analysed).
The researchers concluded their study by suggesting that their data indicates that gingipain inhibitor treatment “will reduce P. gingivalis infection in the brain and slow or prevent further neurodegeneration and accumulation of pathology in AD patients“.
Wow! So this is a very recent research report. I suppose we’ll have to wait to see anything happen clinically about this?
How wrong you are.
Many of the researchers involved in this new report work at a Californian company called Cortexyme.
 In the final part of their research report described in today’s post, the researchers tested a compound called COR388. Oral administration of COR388 twice daily in mice resulted in reductions in porphyromonas gingivalis levels, reductions in beta amyloid levels, and inflammatory marker levels in an established porphyromonas gingivalis brain infection.
In the final part of their research report described in today’s post, the researchers tested a compound called COR388. Oral administration of COR388 twice daily in mice resulted in reductions in porphyromonas gingivalis levels, reductions in beta amyloid levels, and inflammatory marker levels in an established porphyromonas gingivalis brain infection.
And while the scientists were trying to get their report published in a peer-reviewed scientific journal, the biotech firm initiated not one, but two Phase I, double blind, placebo-controlled clinical trials of COR388 in healthy adults and people with Alzheimer’s (Click here and here to read more about the details of those studies).
In October 2018, Cortexyme announced the results of their Phase I trial at the 11th Clinical Trials in Alzheimer’s Disease Conference.
 Source: Aging-news
Source: Aging-news
The results suggest that the compound is safe and well tolerated in healthy older volunteers and Alzheimer’s patients treated with a range of doses for up to 28 days. They also reported that COR388 was detectable in the cerebral spinal fluid along with fragments of porphyromonas gingivalis DNA.
Importantly, while the study was not powered for assessing efficacy, individuals with Alzheimer’s treated with COR388 demonstrated positive trends across several cognitive tests. Given that the trial was only 28 days long, I’m not sure that this really means much, but it is interesting given that the study was double blind – participants were not aware of which treatment they were receiving (Click here to read the press release about the results).
SInce then, Cortexyme has initiated a large Phase II/III clinical trial of COR388 in 573 people with mild to moderate Alzheimer’s. The GAIN (GingipAIN Inhibitor for Treatment of Alzheimer’s Disease) Trial will involve the participants being randomly assigned to one of two doses of COR388 (twice daily 40mg or 80mg), or a placebo treatment. The study will be conducted across more than 90 sites in the United States and Europe (Click here to read more about the details of the study and click here to read the press release).
That’s a big study. Has anyone else ever found a similar association between Alzhiemer’s and Porphy…romo…whatever?
So this is where the story gets a little grey.
You see, the answer is yes, but the results are mixed.
Que?
Back in 2013, there was a report demonstrating the presence of Porphyromonas gingivalis in some of the brain samples collected from people who passed away with Alzheimer’s. 4 out of 10 Alzheimer’s cases were positive, but all of the control samples were negative (Click here to read more about this). This suggests that Porphyromonas gingivalis may not necessarily be associated with all cases of Alzheimer’s.
In addition, three independent research groups have demonstrated that Porphyromonas gingivalis can exacerbate brain Aβ deposition and cognitive issues in mice (Click here, here and here to read those studies).
But a more recent study reported that continuous brain exposure of porphyromonas gingivalis resulted in cardiac injury, but did NOT result in enhanced cognitive impairment in an Alzheimer’s mouse model (Click here to read more about this).
Thus, the idea that this one oral bacteria could explain all cases of dementia may not be exact.
All of this is great, but what does any of it have to do with Parkinson’s?
There has been a long history of viral theories for Parkinson’s (Click here and here to read previous SoPD posts on this topic).
Parkinson’s is characterised in the brain by the clustering (or aggregation) of a protein inside cells called alpha synuclein. It collects into small circular structures called Lewy bodies (Click here to read more about this).
 Parkinson’s associated alpha synuclein. Source: Nature
Parkinson’s associated alpha synuclein. Source: Nature
Given the intracellular nature of alpha synuclein aggregation, the author of this blog has long harboured a theory of a viral/microbial component to Parkinson’s. And there is evidence to suggest that alpha synclein protein has anti-viral properties, such as this report:

Title: Alpha-Synuclein Expression Restricts RNA Viral Infections in the Brain.
Authors: Beatman EL, Massey A, Shives KD, Burrack KS, Chamanian M, Morrison TE, Beckham JD.
Journal: J Virol. 2015 Dec 30;90(6):2767-82. doi: 10.1128/JVI.02949-15.
PMID: 26719256 (This article is OPEN ACCESS if you would like to read it)
David Beckham (not the football player) and his research colleagues introduced West nile virus to brain cells grown in cell culture and they observed an increase in alpha synuclein production. They also found that the brains of people with West nile infections had increased levels of alpha synuclein.
The researchers then injected West Nile virus into both normal mice and genetically engineered mice (which produced no alpha synuclein) and they found that the genetically engineered mice which produced no alpha synuclein died quicker than the normal mice. They reported that there was an almost 10x increase in viral production in the genetically engineered mice. This suggested to them that alpha synuclein may be playing a role in protecting cells from viral infections.
More recently, this property has been independently varified:
 Title: Holocranohistochemistry enables the visualization of α-synuclein expression in the murine olfactory system and discovery of its systemic anti-microbial effects.
Title: Holocranohistochemistry enables the visualization of α-synuclein expression in the murine olfactory system and discovery of its systemic anti-microbial effects.
Authors: Tomlinson JJ, Shutinoski B, Dong L, Meng F, Elleithy D, Lengacher NA, Nguyen AP, Cron GO, Jiang Q, Roberson ED, Nussbaum RL, Majbour NK, El-Agnaf OM, Bennett SA, Lagace DC, Woulfe JM, Sad S, Brown EG, Schlossmacher MG.
Journal: J Neural Transm (Vienna). 2017 Jun;124(6):721-738.
PMID: 28477284 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers infected mice with a reovirus (reovirus-T3D) which is lethal. The mice they injected had different levels of alpha synuclein. They used:
- Normal mice (with two copies of the alpha synuclein gene, SNCA)
- SNCA +/- mice (with just one copy of the alpha synuclein gene, SNCA)
- SNCA -/- mice (with no copies of the alpha synuclein gene, SNCA)
A gene is a section of DNA that provides the instructions for producing a protein, and the alpha synuclein gene is called SNCA. There are two copies of each gene in your DNA (mother nature’s insurance policy in case one copy is defective), and if both SNCA genes are removed there will be no alpha synuclein protein.
Similar to David Beckham’s report, the researchers found that mice with no alpha synuclein protein died faster than mice with alpha synuclein.
 Source: PMC
Source: PMC
And in addition to this anti-viral property, alpha synuclein has also been shown to have anti-microbial properties (Click here to read more about this).
As far as I’m aware, there is no association between Parkinson’s and Porphyromonas gingivalis (happy to be corrected on this).
Hang on a second. What about all of the alpha synuclein antibody clinical trials? If alpha synuclein is involved in antimicrobial activity, then isn’t it bad to remove it from the brain?
No.
Firstly, while this whole idea of anti-viral/microbial properties for beta amyloid and alpha synuclein is very interesting and compelling, it must be remembered that it is all based on a rather limited amount of pre-clinical data. Thus, it is all basically hypothetical (at best) for now.
In addition, there is some serious potholes in the idea. For example, people with multiple copies of the beta amyloid gene or alpha synuclein gene are actually more likely to develop Alzheimer’s or Parkinson’s (respectively). The anti-viral/microbial theory would suggest that they should be less likely to. So how we explain this confounder is unclear.
Second, the alpha synuclein antibody clinical trials (Click here to read more about this) are targetting a toxic form of the protein floating around outside of cells, so it should not affect any potential internal ‘defensive properties’ of this protein. The logic of the alpha synuclein antibody approach is still sound, and based on a lot more data than we have for the anti-viral/microbial idea.
But if the beta amyloid antibody clinical trials didn’t work for Alzheimer’s, why should an alpha synuclein antibody work for Parkinson’s?
Because they are very different conditions.
The alpha synuclein antibody trials are based on the idea that a toxic form of this protein is being passed between cells and this is how Parkinson’s is slowly progressing. This is very different to the beta amyloid theory.
There is also the argument that the beta amyloid antibody approach may have worked for some participants, but not enough to see an overall effect. This begs the question: what was unique about those ‘responders’ compared to non-responders. I would like to see someone do a deep dive into all of the data surrounding those clinical trials and identify all of the responders to the treatment.
Investigating the characteristics of responders should be an important focus of all post-clinical trial activities.
Ok, so what does it all mean?
A Californian biotech firm has not only found an association between the neurodegenerative condition of Alzheimer’s and a bacteria that causes gum disease, but they have also developed (and are clinically testing) a drug that stops this bacteria. Whether the bacteria is actually involved in Alzheimer’s and whether the drug can slow or stop the cognitive decline resulting from this form of dementia is yet to be determined, but this is certainly an exciting development in the world of neurodegenerative research.
The hope of the author of this blog is that a thorough analysis of postmortem brain tissue will now be made of people that passed away from many different types of neurodegenerative conditions (not just Parkinson’s). While there is still a lot of ‘dark matter’ in our knowledge of the viral/microbial world (“we still know only a small fraction of the viruses that exist” – Source), we are learning more every day and some of these new organisms could be influential in human conditions.
But going back to my point at the start regarding associations: causation is the hardest thing to prove in science. And given the variability in symptoms/features between individuals with a condition like Parkinson’s, whether a particular virus/microbe is involved with all cases is questionable and will be difficult to prove. But I like this alternative way of looking at neurodegenerative conditions, and I will be following the Cortexyme trial with great interest.
EDITOR’S NOTE – The company Cortexyme mentioned in this post is about to become a publicly traded company. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. Neither Cortexyme or any associated parties have requested that this material be produced, nor has the author had any contact with the company or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from Cortexyme


Idea: What if this gingivitis bacterium is directly inciting an inflammatory feedback process in the brain (just as, say, LPS does) that kills neurons there directly, causing them to dump their contents, including amyloid, into intracellular space? That could explain the increase in amyloid, which then might help entrap the bacterium, but with insufficient efficiency to prevent amyloid buildup and the formation of plaques.
So then the amyloid would not be generated as a defense against the bacterium, but rather as a result of the destruction of neurons that was initiated by the bacterium.
This hypothesis seemed consistent with the fact that “people with multiple copies of the beta amyloid gene or alpha synuclein gene are actually more likely to develop Alzheimer’s,” because that would just increase the amount of amyloid buildup, increasing the inflammatory response.
LikeLike
“intracellar space” above should read “intracellular space.”
LikeLike
“intracellar space” above should read “intercellular space.” Some kind of autocorrect going on, sorry.
LikeLike
Great post about Porphyromonas gingivalis. This may be a important milestone in the battle against Alzheimer, and Parkinsons was well for that matter. Gingipain levels might be a marker of the bacteria’s activity.
LikeLike
https://www.frontiersin.org/articles/10.3389/fnagi.2019.00210/full
LikeLike