|
With the recent announcement that the STEADY-PD III/Isradipine clinical trial did not reach its primary end point (that of slowing the progression of Parkinson’s), the winds of change have shifted with calls for a focus on biomarkers and better treatments, rather than disease modification. Recently, researchers at Michigan State University have reported a novel experimental gene thearpy method for dealing with one of the most debilitating aspects of Parkinson’s – dyskinesias. Ironically, their approach involves the same calcium channels that Isradipine blocks. In today’s post, we will look at what dyskinesias are, what gene therapy is, and how this new approach could be useful for people currently burdened by these involutary movements.
|
 Dyskinesia. Source: JAMA Neurology
Dyskinesia. Source: JAMA Neurology
There is a normal course of events following a diagnosis of Parkinson’s.
Yes, I am grossly over-generalising.
And no, I’m not talking from personal experience (this is based on listening to a lot of people), but just go with me on this for the sake of discussion.
First comes the shock of the actual diagnosis. For many it is devastating news – an event that changes the course of their lives. For others, however, the words ‘you have Parkinson’s‘ can provide a strange sense of relief that their current situation has a name and gives them something to focus on.
This initial phase is usually followed by the roller coaster of various emotions (including disbelief, sadness, anger, denial). It depends on each individual.
 The emotional rollercoaster. Source: Asklatisha
The emotional rollercoaster. Source: Asklatisha
And then comes the period during which many will try to familiarise themselves with their new situation. They will read books, search online for information, join Facebook groups (Click here for a good one), etc.
That search for information often leads to awareness of some of the realities of the condition.
And one potential reality that causes concern for many people (especially for people with young/early onset Parkinson’s) is dyskinesias.
What are dyskinesias?
Dyskinesias (from Greek: dys – abnormal; and kinēsis – motion, movement) are a category of movement disorders that are characterised by involuntary muscle movements. And they are certainly not specific to Parkinson’s.
But in the case of Parkinson’s, dyskinesias have generally been believed to be associated with long-term use of Levodopa (also known as Sinemet or Madopar).

Sinemet is Levodopa. Source: Drugs
NOTE: Long-term use of Levodopa is not a certainty for developing dyskinesias, but there is an association. It will differ from person to person.
How do dyskinesias develop in Parkinson’s?
There is a lot of debate over this topic, but there are some basic details that researchers generally tend to agree on.
Before being diagnosed and beginning a course of Levodopa, the locomotion parts of the brain in a person with Parkinson’s gradually becomes more and more inhibited. This increasing level of inhibition results in the slowness and difficulty in initiating movement that characterises this condition.
A person with Parkinson’s may want to move, but they can’t – they are inhibited. In effect, they are akinetic (from Greek: a-, not, without; and kinēsis – motion).

Drawing of an akinetic individual with Parkinson’s, by Sir William Richard Gowers
Source: Wikipedia
Levodopa (or L-DOPA) tablets provide the brain with the precursor to the chemical dopamine. Dopamine producing cells are lost in Parkinson’s, so replacing the missing dopamine is one way to treat the motor features of the condition. Simply giving people pills of dopamine is a non-starter though: dopamine is unstable, breaks down too quickly, and (strangely) has a very hard time getting into the brain (it is blocked by the protective layer called the blood-brain-barrier). Levodopa, on the other hand, is very robust and has no problem entering the brain.
Once inside the brain, Levodopa is quickly converted into dopamine. It is changed into dopamine by an enzyme called DOPA decarboxylase, and this change rapidly increases the levels of dopamine in the brain, allowing the locomotion parts of the brain to function more normally.

The chemical conversion of L-DOPA to dopamine. Source: Nootrobox
In understanding this process, it is important to appreciate that when an Levodopa tablet is consumed and Levodopa enters the brain, there is a rapid increase in the levels of dopamine. This ‘spike’ in the supply of dopamine will last for the next few hours, before the dopamine is eventually used up.
As the effects of the Levodopa tablet wear off, another tablet will be required. This use of multiple Levodopa pills across the day gives rise to a wave-like shape to the dopamine levels in the brain over the course of the day (see the figure below). The first pill in the morning will quickly lift the levels of dopamine enough that the individual will no longer feel akinetic. This will allow them to be able to function with normal controlled movement for several hours before the Levodopa begins to wear off. As the Levodopa wears off, the dopamine levels in the brain drop back towards levels that will leave the person feeling akinetic and at this point another Levodopa tablet is required.
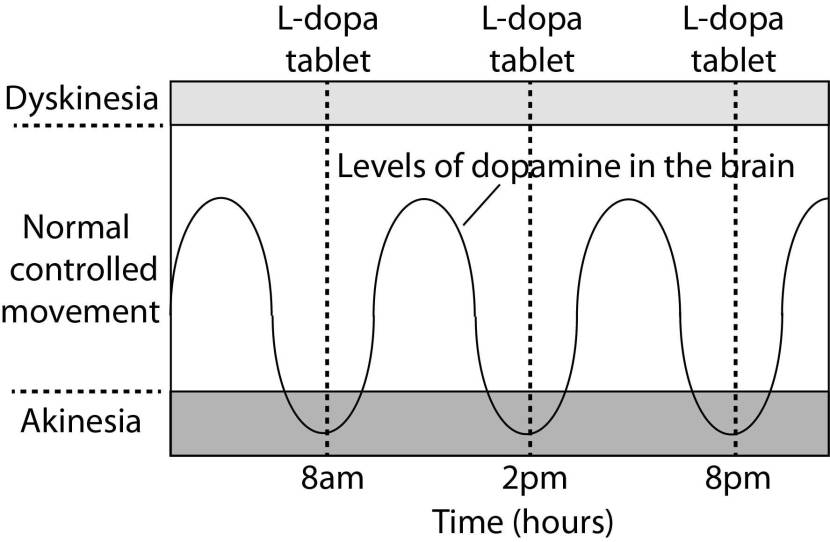
A hypothetical illustration of dopamine levels over a day
After several years of Levodopa use, many people with Parkinson’s will experience a weaker response to each tablet. They will also find that they have more time during which they will be unable to move (exhibiting akinesia). This is simply the result of the progression of Parkinson’s – Levodopa treats the motor features of the condition but only hides/masks the fact that the disease is still progressing.
To combat this shorter response time, the dose of Levodopa is usually increased. This will result in increasing levels of dopamine in the brain (as illustrated by the higher wave form over time in the image below). Gradually it will take more Levodopa medication-induced dopamine to lift the individual out of the akinetic state.

Again this illustration is hypothetical (situation differs between individuals)
This increasing of Levodopa dosage, however, results in too much dopamine being present in the brain at times. And this situation is often associated with the gradual development of abnormal involuntary movements that appear when the levels of Levodopa induced dopamine are the highest.
These are the dyskinesias.
Are there different types of dyskinesias?
Yes there are.
Dyskinesias have been broken down into many different subtypes, but the two main types of dyskinesia are:
- Chorea – these are involuntary, irregular, purposeless, and unsustained movements. To an observer, Chorea will look like a very disorganised/uncoordinated attempt at dancing (hence the name, from the Greek word ‘χορεία’ which means ‘dance’). While the overall activity of the body can appear continuous, the individual movements are brief, infrequent and isolated. Chorea can cause problems with maintaining a sustained muscle contraction, which may result in affected people dropping things or even falling over.
- Dystonia – these are sustained muscle contractions. They often occur at rest and can be either focal or generalised. Focal dystonias are involuntary contractions in a single body part, for example the upper facial area. Generalised dystonia, as the name suggests, are contraction affecting multiple body regions at the same time, typically the trunk, one or both legs, and another body part. The intensity of muscular movements in sufferers can fluctuate, and symptoms usually worsen during periods of fatigue or stress.
How is dyskinesia currently treated?
Generally with medication or deep brain stimulation (or DBS – Click here to read more about ‘DBS’).
Recently, however, researchers have been exploring alternative approaches to helping alleviate dyskinesias, including the use of gene therapy.
What is gene therapy?
Gene therapy is an experimental treatment approach that involves treating medical conditions with DNA rather than drugs.
 Source: Baltimoresun
Source: Baltimoresun
Gene therapy basically involves introducing a new piece of DNA or replacing a faulty piece of DNA within a population of cells. DNA, as you may remember from high school science class provides the instructions for making proteins in the a cell and these proteins are the bits that actually do stuff.
By introducing a new piece of DNA into a cell, the cell can start to produce a functioning protein that it may not normally produce. In some diseases, a cell may normally produce a particular protein, but because the genetic instructions in the DNA (a section of the DNA called a gene) for that protein have a small error (a genetic mutation), a non-functioning version of the protein is actually being produced. The introduction of the new correct (functioning) version of that piece of DNA (or gene) into a cell can start the production of a functional version of the protein.
 Gene therapy. Source: yourgenome
Gene therapy. Source: yourgenome
Alternatively, a gene can be introduced into a cell which would cause the cell to produce a protein that that cell usually does not produce. This sort of approach is being used in gene therapy for cancer, where ‘suicide genes’ are being introduced into cancer cells. These cause the cancer cell to die, by initiate an auto-destruct sequence resulting in cell death (a process called apoptosis). Another approach the cancer field is using is introducing a gene into cancer cells that cause a protein to be produced on the surface of the cancer cell that attracts the attention of the immune system. This ‘marker gene’ causes the immune system to attack the cancer cell, resulting in the death of the cancer cell.
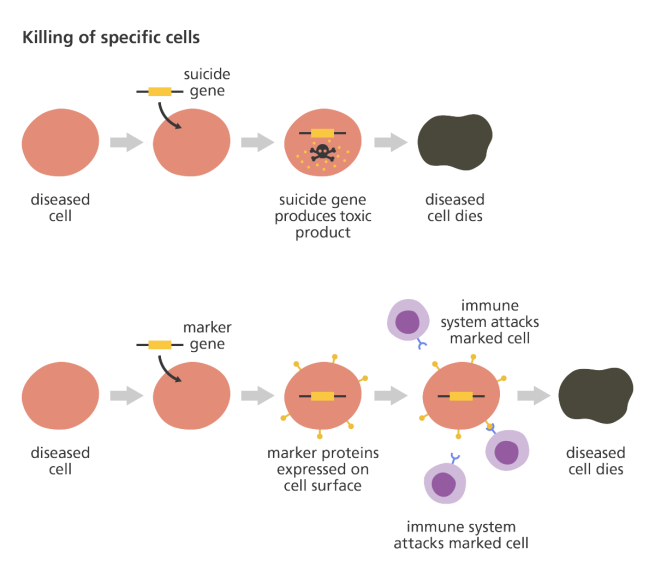 Source: yourgenome
Source: yourgenome
Taking this approach one step further, we can take sections of DNA that contain the genes involved with the production of a proteins that would be beneficial for the cell, such as GDNF. By then injecting a virus with the DNA for GDNF into the brain, we can produce GDNF in any infected cells (it’s slightly more complicated than that, but you get the basic idea). And such a gene therapy approach has recently been tested in a clinical trial (Click here to read more about this).

Gene therapy for Parkinson’s disease. Source: Wiki.Epfl
I’m sorry, but did you say viruses?
Yes, if you remove the viral DNA from inside a virus and replace it with something useful, then a virus becomes a very useful biological delivery system. Far superior to anything we humans have devised thus far. Viruses are easy to produce and manipulate, and they can even be engineered to target specific cell types.
And these viruses have been engineered NOT to replicate. Their ability to replicate themselves has been completely removed.
They deliver the proposed DNA and that is all.
Ok, so how have researchers been using gene therapy to deal with dyskinesias?
It must be emphasized that this research is still very experimental, but recently this research report was published:
 Title: Genetic silencing of striatal CaV1.3 prevents and ameliorates levodopa dyskinesia
Title: Genetic silencing of striatal CaV1.3 prevents and ameliorates levodopa dyskinesia
Authors: Steece-Collier K, Stancati JA, Collier NJ, Sandoval IM, Mercado NM, Sortwell CE, Collier TJ, Manfredsson FP.
Journal: Mov Disord. 2019 Apr 19. [Epub ahead of print]
PMID: 31002755 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers were interested in using gene therapy to prevent the development of Levodopa-induced dyskinesias and reduce already established dyskinesias.
To do this, they focused on calcium channels.
What are calcium channels?
Everyone has grown up hearing that calcium is important for your bones, but above and beyond that calcium is critical for the normal functioning of your entire nervous system.
Few if any neural signals would get transmitted in the absense of calcium.
Across the cell membrane (the wall of each cell), there is a ‘concentration gradient of ions’ (an ion is a molecule with a net electric charge due to the loss or gain of one or more electrons). This gradient simply means that there are more of certain chemical elements outside the cell than inside the cell (or vice versa). You can see this in the image below:
Source: Wikipedia
When a neuron is at rest, there is a high concentration of sodium (Na+) ions and chloride (Cl-) ions outside of the neuron (in the extracellular fluid) compared the situation inside the cell (the intracellular fluid) where there is a high concentration of potassium (K+) ions.
 Source: Washington
Source: Washington
When a neuron is transmitting a signal along its branches, this balance is reversed – sodium ions and chloride ions rush inside of the neuron, while potassium moves out. After the impulse has passed, there are active mechanisms that return the balance to normal (high concentration of sodium ions and chloride ions outside of the cell, etc).
The in flow of sodium and cholride into neurons activates (voltage-dependent) calcium channels, which open up and allow calcium to come flowing into the neuron. Calcium is very important for the release of chemical messengers (like neurotransmitters). When calcium channels are blocked, neurotransmitter release is inhibited.
The critical thing to understand in the case of an action potential is that without calcium, communication between neurons becomes harder. And this is why calcium is so important in our diet.
 Foods containing high levels of calcium. Source: Animalsaustralia
Foods containing high levels of calcium. Source: Animalsaustralia
I see. But what do calcium channels have to do with Parkinson’s or dyskinesias?
Many years back, researchers noted that people being treated with a calcium channel blocker medication that specifically targeted a specific type of calcium channel (called L-type calcium channels) had a reduced risk of developing Parkinson’s:
 Title: L-type calcium channel blockers and Parkinson disease in Denmark.
Title: L-type calcium channel blockers and Parkinson disease in Denmark.
Authors: Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S.
Journal: Ann Neurol. 2010 May;67(5):600-6.
PMID: 20437557 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers collected the medical records of 1,931 people in Denmark who were diagnosed with Parkinson’s between 2001 and 2006. These records were age and sex matched to 9,651 records of healthy controls from the same register. After analysing all of the records, the investigators found that people prescribed with a type of medication called a L-type calcium channel blocker (dihydropyridines) were 27% less likely to develop Parkinson’s.
This finding supported a previous study that found a similar result (Click here to read more about that) and the same result was independently replicated a couple of years later (Click here to read that report).
And this finding was also supported by postmortem analysis of the Parkinsonian brain:
 Title: Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins.
Title: Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins.
Authors: Hurley MJ, Brandon B, Gentleman SM, Dexter DT.
Journal: Brain. 2013 Jul;136(Pt 7):2077-97.
PMID: 23771339 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers looked at where in the human brain the L-type calcium channels were present and in what concentration. They used sections of postmortem brain to carry out this analysis, looking at both healthy specimens as well as those from people who passed away with Parkinson’s.
In the normal brains, the distribution of the L-type calcium channels and certain calcium-binding proteins was not associated with regions of the brain that are prone to the selective neurodegeneration that is seen in Parkinson’s. But the investigators observed a very different picture in the Parkinsonian brains.
Increased levels of L-type calcium channels and the calcium-binding proteins were found throughout the brains of people who passed away with Parkinson’s. Even in cases of early Parkinson’s (in people who passed away shortly after being diagnosed), increased levels of L-type calcium channels were even found in the cerebral cortex – a part of the brain largely unaffected in Parkinson’s.
These findings lead the researchers to conclude that “disturbed calcium homeostasis” may be “an early feature of Parkinson’s disease and not just a compensatory consequence to the neurodegenerative process”.
And the dyskinesia association?
It had previously been demonstrated that calcium channel blockers can also reduce dyskinesias in models of Parkinson’s (Click here and here to read more about this).
So the researchers behind the gene therapy report decided to try and block calcium channels on a more permanent basis (rather than the period nature of taking medication 2-3 times per day).
Specifically, they sought to reduce levels of a particular L-type calcium channel called CaV1.3. They did this using carefully engineered viruses that infect cells with a microRNA for CaV1.3.
What is a microRNA?
MicroRNA are a small strings of RNA (containing about 22 nucleotides) found in plants, animals and some viruses. They are non-coding RNA – which means they do not provide the instructions for making protein – but rather, they play impoartant functions in regulating levels of other proteins. MicroRNAs are often mirror copies of coding RNAs, and when the microRNA binds to their ‘mirror’ protein coding RNA they can stop that RNA from making more protein.
 MicroRNA blocking the production of protein. Source: Integragen
MicroRNA blocking the production of protein. Source: Integragen
For those interested in learning more about microRNA – watch this video which explains them very well:
Given the ability to stop (or silence) the production of specific proteins, scientists have harnessed this ability of microRNAs and they have started designing microRNAs to try and silence many different types of proteins for therapeutic benefit, such as the CaV1.3 calcium channel.
I see. So what happened when the researchers used microRNA to silence CaV1.3 calcium channel in models of dykinesia?
The researchers started their experiment by injecting rats with the virus producing microRNA for CaV1.3. The virus was injected into a region of the brain called the striatum, which is where dopamine neurons release much of their dopamine. Next they induced a model of Parkinson’s using a neurotoxin (6-OHDA) and then they started treating the animals with high doses of Levodopa to cause Levodopa induced dyskinesias (see panel A in the image below for a schematic of this study design).
The researchers found that rats who were injected the virus carrying the CaV1.3 microRNA did not develop dyskinensias… at all! If you look at panel B in the image below, you can see a black line of the control rats (no gene therapy, but model of Parkinson’s with Levodopa induced dyskinesias) rapidly develop the dyskinesias with increasing severity as the dose of Levodopa is increased. The red line bouncing along the bottom of the graph are the gene therapy treated animals with no dyskinesias (CaV1.3 microRNA virus followed by model of Parkinson’s with Levodopa induced dyskinesias).
 Source: Wiley
Source: Wiley
This was a really impressive result!
But the researchers wanted to take this finding one step further and see if they can’t stop established dyskinesias – that is to say, can the gene therapy approach work after dyskinesias have already developed?
So the investigators repeated the experiment, except this time they induced a model of Parkinson’s using a neurotoxin (6-OHDA) and treating the animals with high doses of Levodopa to cause Levodopa induced dyskinesias before they treated some of the animals with the gene therapy treatment (see panel A in the image below for a schematic of this study design).
As you can see from panel B of the image below, the red line of the gene therapy treated animals starts to reduce from about 2 weeks post-gene therapy treatment. The gene therapy treament (microRNA reduction of CaV1.3) resulted in a significant reversal of established, severe Levodopa induced dyskinesias.
 Source: Wiley
Source: Wiley
And remarkably, the researchers demonstrated that this CaV1.3 silencing by gene therapy did not interfere with motor response to Levodopa. That is to say, Levodopa retained its ability to improve motor function in the rodent models of Parkinson’s despite the gene therapy treatment.
If this experimental gene therapy treatment can be translated into humans, it could represent an impressive new method of treating Levodopa-induced dyskinesias.
And the researchers who conducted this research have recently been awarded a large research grant to further develop this work (Click here to read more about this).
 Dr Fredric Manfredsson (left), Prof Timothy Collier (center) & Prof Kathy Steece-Collier (right). Source: MSU
Dr Fredric Manfredsson (left), Prof Timothy Collier (center) & Prof Kathy Steece-Collier (right). Source: MSU
I’m thinking of getting deep brain stimulation to help control my dyskinesias. Should I wait for this new approach?
Please understand that this new method is still very experimental. It may not translate to humans (there may be major differences between rats and humans).
In addition, this new method is not tunable like deep brain stimulation. By this, I mean that there is no volume control for gene therapy. Once it is injected and the virus infects a cell there is no way of increasing or decreasing the amount of microRNA being produced.
One of the benefits of deep brain stimulation (or DBS) is the ability to programme the level of stimulation (Click here to read a previous SoPD post on DBS).
So what does it all mean?
The title of today’s post is partly a salute to Tom Isaacs – one of the co-founders of the Cure Parkinson’s trust – who tried to offer a humourous label to what is a very serious and debilitating aspect of Parkinson’s.
Dyskinesias are one of the greatest concerns for some members of the Parkinson’s community. They are a distressing aspect of an already distressing condition. But there is a great deal of research being conducted on this area, particularly as clinical trials of potentially disease modifying therapies give negative results (see the recent Isradipine STEADY-PD trial) and there are calls for the research community to shift more focus on to improving quality of life for folks living with the condition.
It is interesting to note that the same calcium channels being blocked in this new gene therapy study were the ones being blocked in the Isradipine STEADY-PD trial. It would be interesting if the researchers behind the STEADY-PD trial could follow up with the participants to see if the 3 years of treatment with a calcium channel blocker Isradipine could causes any kind of delay in the emergence of dyskinesias in the study cohort.
Such information could be very useful if this new gene therapy approach is to be eventually taken into clinical trials.
The banner for today’s post was sourced from cellculturedish


Fascinating post, Simon! Thank you for that.
One thing I’m wondering about is the efforts to smooth out the available dopamine levels, like the accordion pill or extended release Sinemet. It seems like, with a proper loading dose and frequent administration of, or extended decay rates of dopamine, those wild swings could be controlled much better than you show in the diagram. With lower amplitude to the oscillation, the off periods and incidence of dyskinesia could both be delayed to much later in the course of the disease. Are we at the limit of our ability to control dopamine levels?
LikeLike
Hi,
many thanks for this exciting post!
I find it interesting and confusing that some calcium channel blockers seem to lower the risk of PD as described here, while other calcium channel blockers, such as cinnarizine, are known to be able to trigger or unmask a Parkinson’s syndrome. One of my own Parkinson’s symptoms first appeared while I was taking cinnarazine, 20 years before I was diagnosed with idiopathic PD. I understand that there are different types of calcium channels, but I still find this phenomenon funny and wonder how it all fits together.
zz
LikeLike
Here’s are the results from the STEADY-PD trial of Isradipine.
https://www.mdedge.com/neurology/article/199907/parkinsons-disease/isradipine-parkinsons-disease-fails-phase-3-study
LikeLike