|
For the last 21 years, the protein alpha synuclein has developed a reputation as public enemy #1 in the world of Parkinson’s. Tiny errors in the DNA that provides the instructions from making the alpha synuclein protein were found to be the first genetic risk factor for the condition, and then the protein itself was found to be present in Lewy bodies – one of the cardinal features of Parkinson’s in the brain. In addition, animal models of Parkinson’s involving the production of high levels of alpha synuclein have demonstrated that this build up of protein can be neurotoxic, and it has been reported that alpha synuclein deposits can appear in cells that had been transplanted into the brains of people with the Parkinson’s. But very recently a new theory (and supporting data) regarding this protein has been proposed, and it paints a slightly different picture. In today’s post we will look at this new theory (and the provided data), and consider what this could mean for our efforts to therapeutically deal with Parkinson’s.
|
 Source: Haikudeck
Source: Haikudeck
Ok, today’s post is diving straight into the science lesson.
No preamble, just good old cell biology.
In almost every cell in your body, there is a structure called the nucleus. It is rather critical to life as we know it, because the nucleus is the vault which holds the blue prints (aka DNA) for making and maintaining a copy of ‘you’.
 The nucleus of a cell. Source: Biologywise
The nucleus of a cell. Source: Biologywise
The nucleus is a very busy area of any cell as it provides the instructions for cellular function. At any point in the day or night, many regions of the DNA are continuously being read and converted in RNA (in a process called transcription).
While DNA is the blue print, RNA is the facsimile of a region of DNA that is used for making a particular protein. While DNA is precious, unique, and must be carefully guarded, the copied version (RNA) is readily disposable. Once produced (or transcribed), each piece of RNA will exit the nucleus and enter an adjoining structure called the endoplasmic reticulum. On a very basic level, the endoplasmic reticulum is where the RNA facsimile of the instructions is used to produce protein.
 Making a protein. Source: Quora
Making a protein. Source: Quora
Some of these newly formed proteins will be released outside of the cell (to send messages to other cells), while some other newly formed proteins will be transported to distant parts of the cell to do specific tasks. But another collection of the newly formed proteins will be shipped back to the nucleus where they will play important roles in maintaining, transcribing, monitoring, or repairing the precious DNA.
Recently published research suggests that the Parkinson’s associated protein alpha synuclein may have a function in the protection of DNA.
Specifically, it appears to be involved with DNA repair.
Remind me again: What is alpha synuclein?
Alpha synuclein sounds like a distant galaxy, but it is one of the most common proteins in our brains. It makes up about 1% of all the protein in a neuron.
How is alpha synclein associated with Parkinson’s?
When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure.
When it is first produced, alpha synuclein will look something like this:
 Alpha synuclein. Source: Wikipedia
Alpha synuclein. Source: Wikipedia
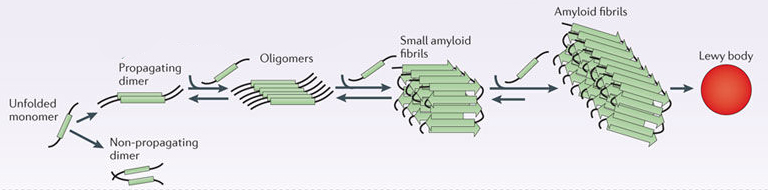
In this form, alpha synuclein is considered a monomer – which is a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). It is believed that alpha synuclein has certain functions as a monomer, but may also have specific tasks as an oligomer.
In Parkinson’s, alpha synuclein will also misfold and aggregate together to form amyloid fibrils.

Microscopic images of monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein that aggregate together, and then go on to form what we call Lewy bodies.
 Parkinson’s associated alpha synuclein. Source: Nature
Parkinson’s associated alpha synuclein. Source: Nature
A Lewy body is referred to as a cellular inclusion, as they are almost always found inside the cell body. They are a characterisitic feature of the Parkinsonian brain.
 A photo of a Lewy body inside of a neuron. Source: Neuropathology-web
A photo of a Lewy body inside of a neuron. Source: Neuropathology-web
It has long been debated whether these Lewy bodies and alpha synuclein protein aggregates are toxic agents in the cause of Parkinson’s, or a desperate attempt at self preservation by an unhealthy cell.
But people with genetic variations in their alpha synuclein gene have an increased risk of developing Parkinson’s (Click here to read more about this). In addition, preclinical models of Parkinson’s suggest that high levels of aggregated alpha synuclein can be toxic (Click here to read more about this).
All of these bits of evidence have led to the idea that alpha synuclein is the bad boy of the Parkinson’s world.
What does alpha synuclein do normally? What function does it have in a healthy cell?
Good question. It appears to have a number of different functions,
In healthy brain cells, correctly constructed alpha synuclein is typically found just inside the surface of membrane surrounding the cell body and in the tips of the branches extending from the cells (in structures called presynaptic terminals which are critical to passing chemical messages between neurons).

Presynaptic terminal (right) releasing chemical messengers to another cell. Source: Truelibido
Recently, however, researchers from Portland (Oregon) has published research suggesting a new role for alpha synuclein, which could be involved with making neurons vulnerable in Parkinson’s.
Here is their report:
 Title: Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders.
Title: Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders.
Authors: Schaser AJ, Osterberg VR, Dent SE, Stackhouse TL, Wakeham CM, Boutros SW, Weston LJ, Owen N, Weissman TA, Luna E, Raber J, Luk KC, McCullough AK, Woltjer RL, Unni VK.
Journal: Sci Rep. 2019 Jul 29;9(1):10919.
PMID: 31358782 (This report is OPEN ACCESS if you would like to read it)
Since it was first discovered, there has been reports demonstrating that alpha synuclein protein is present synaptic regions of neurons, but there have been studies suggesting that it is also in the nucleus (Click here for an early example). Recently, there has been a growing body of evidence indicating that not only is the protein present in the nucleus, but that it also has biological effects there (Click here for an example).
In this new study, the researchers found that alpha synuclein protein in the nucleus was located righ next to protein associated with DNA repair proteins (such as histone 2 A.X and poly-ADP ribose (or PAR – which we have discussed in a previous SoPD post – click here to read that post).
 Source: PMC
Source: PMC
This precise location got the investigators wondering if alpha synuclein might be involved in DNA repair mechanisms of cells.
What is DNA repair?
Our bodies are constantly under attack for various environmental factors (viruses, UV radiation from the sun, too much time on Facebook, etc). When individual cells are put under stress, there will quite often be damage to the DNA in that cell. DNA is robust, but as there is 3 billion base pairs (resulting in 2 meters of DNA) in each cell, there is plenty of opportunity for damage to be done.
Different forms of stress that lead to DNA damage. Source: Sierraoncology
And given the importance of DNA, mother nature has very wisely devised several very clever systems of DNA repair to maintain the integrity of this precious molecule. But the correct functioning of these DNA repair facilities is critical to healthy living. Disruption of the DNA repair processes can lead to cell death.
So given the presence of alpha synuclein right next to some of the key players in DNA repair, the researchers decided to have a look at whether removing alpha synuclein would comprimise the DNA repair response.
They found that under normal conditions (eg. no stress to the cells), there was no difference in the DNA repair response between cells with and without alpha synuclein. But when the researchers caused DNA damage (using a chemical called bleomycin), they reported that cells that produce alpha synuclein protein were able to repair DNA damage more quickly than cells without alpha synuclein, suggesting that alpha-synuclein may facilitates the DNA repair response.
They also found that alpha synuclein in the nucleus can directly attach (or bind) to DNA (which supports previous research on this topic – click here to read more about this), and that it is rapidly recruited to sites of DNA breaks – where the chain of DNA has physically broken and needs to be reconnected.
Considering the importance of DNA repair to normal cellular well being, the investigators next decided to explore this novel function of alpha synuclein in the context of Parkinson’s. Interestingly, in both a mouse model of Parkinson’s (the preformed fibril model) and in post mortem human brain tissue from people who passed away with Parkinsons, the researchers found that alpha synuclein pathology was associated with evidence of increased DNA damage.
 Source: PMC
Source: PMC
All of these results suggested to the researchers that when alpha synuclein is clustered or aggregated together (and forming Lewy bodies) in affected neurons, this leads to decreases in levels of the protein in the nucleus. And as a result of this reduction in levels of alpha synuclein in the nucleus, there is more risk of DNA damage.
The investigators concluded that further research is required to better understand the role of alpha synuclein protein in DNA repair functions, and this could help to facilitate the development of new therapeutic targets for multiple forms of neurodegeneration (not just Parkinson’s).
But hang on a second. There are ongoing clinical trials that are trying to clear the brain of toxic alpha synuclein. If reducing alpha synuclein results in more DNA damage, are those clinical trials going to make the situation worse for Parkinson’s?
My guess here is “No they won’t”.
But we really can not make that conclusion based on this new data.
Firstly, these new results need to be independently replicated before we can get too excited about this novel potential function for alpha synuclein. While these results are very interesting and potentially transformational for our understanding of alpha synuclein, replication/validation and expansion of the finding needs is required.
Second, most of the immunotherapy approaches that are being clinically tested (which we have previously discussed on the SoPD – click here and here to read examples) are focused on clearing the toxic form of alpha synuclein as it is being passed between cells. That is, in the extracellular space. They will have little impact on what is actually happening inside the cells. These immunotherapy approaches are trying to slow the progression or spread of Parkinson’s by grabbing the toxic form of alpha synuclein as it hops from one cell to the next.
 And even the drugs that target alpha synuclein aggregates within cells (such as Anle138b – click here to read more about this) are unlikely to make the situation worse, as they are primarily designed to break up protein aggregates – which should in theory increase the amount of alpha synuclein floating around in a cell (possibly improving the situation for DNA repair?!?). And this idea is supported by preclinical models that demonstrate these experimental therapies can rescue cells in models of Parkinson’s.
And even the drugs that target alpha synuclein aggregates within cells (such as Anle138b – click here to read more about this) are unlikely to make the situation worse, as they are primarily designed to break up protein aggregates – which should in theory increase the amount of alpha synuclein floating around in a cell (possibly improving the situation for DNA repair?!?). And this idea is supported by preclinical models that demonstrate these experimental therapies can rescue cells in models of Parkinson’s.
Just understand that most of the therapies being clinically tested for alpha synuclein are targeting the aggregated form of the protein, and leaving the normal (monomeric) alpha synuclein alone. Thus, the alpha synuclein protein should be able to access the nucleus and repair DNA (if that is what it is doing).
So what does it all mean?
Two things I love about science:
1. The re-hashing old ideas: I am a big fan of novel ways of looking at old problems, particularly new ideas that destroy dogma and point us in an alternative direction. Sometimes these “game changers” are wrong, and additional research points out where we were mistaken in our interpretation of the situation/data. But on other occasions these new ideas have opened up new horizons and left our old thinking behind.
There has been accumulating evidence suggesting that the Parkinson’s associated protein alpha synuclein is present in the nucleus of cells, and very recent research suggests that the protein may be involved with DNA repair. This is a very compelling idea, but it raises some intriguing questions about the nature of Parkinson’s.
For example, if alpha synuclein is involved in DNA repair, why are people with high levels of the protein (SNCA triplication) more vulnerable to Parkinson’s? With more alpha synuclein, should they not have less DNA damage?
2. The way a good piece of research will always leave you with more questions than answers.
The banner for today’s post was sourced from Helix


This reminds me of the two sides of NF-kB: yin and yang. A necessity during early growth & development phases but a “pain” when it amps up an inflammatory pathway.
LikeLike
Thanks for this article Simon, a really useful explanation of how the cell works and also how it can malfunction.
LikeLike
Thank you so much for this blog. You put all the relevant knowledge/research inside and then explain to us non-experts so that we can understand. Your blog saved me countless hours of google searching in the wild west of the internet.
LikeLike