|
This week exciting new research from a collaboration between Chinese researchers and scientists at the University of Iowa has pointed towards a clinically-available, generic drug that could be re-purposed for Parkinson’s. The researchers found a drug called Terazosin – which is used for the treatment of enlarged prostates and high blood pressure – can boost energy production in neurons, and also rescue multiple preclinical models of Parkinson’s (including human cell cultures). Most intriguing, however, was their discovery that people taking Terazosin (or similar drugs) have a reduced incidence of Parkinson’s, and people with Parkinson’s who take Terazosin seem to have less disease progression. In today’s post, we will look at what Terazosin is, how it functions, what this new research suggests, and how the finding is being taken forward.
|
 Reader questions. Source: Yoursalesplaybook
Reader questions. Source: Yoursalesplaybook
So I have had a few inquiries over the last 24 hours.
Lots of questions.
A wee bit of interest in some recent Parkinson’s associated research.
It seems that there was a bit of excitement generated by press releases regarding new research from a group of researchers in China and the University of Iowa suggesting that a commonly used blood pressure and prostate treatment called Terazosin not only had beneficial effects in multiple models of Parkinson’s, but also reduced the incidence of Parkinson’s in people taking the drug.
 Terazosin. Source: Wikipedia
Terazosin. Source: Wikipedia
Here is the study in question:
 Title: Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases.
Title: Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases.
Authors: Cai R, Zhang Y, Simmering JE, Schultz JL, Li Y, Fernandez-Carasa I, Consiglio A, Raya A, Polgreen PM, Narayanan NS, Yuan Y, Chen Z, Su W, Han Y, Zhao C, Gao L, Ji X, Welsh MJ, Liu L.
Journal: J Clin Invest. 2019 Sep 16. pii: 129987.
PMID: 31524631 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers investigated the properties of a drug called terazosin in various models of Parkinson’s.
What is terazosin?
Terazosin is a drug that is used to treat high blood pressure (hypertension) and it is also used in men to treat the symptoms of an enlarged prostate (a condition called benign prostatic hyperplasia – or BPH).
It is an α1-adrenergic receptor antagonist.
Hold up! What on Earth does any of that mean? What is a receptor antagonist?
On the surface of a cell, there are lots of small molecules (called receptors) which act as switches for certain biological processes to be initiated. Receptors will wait for a particular protein to come along and bind to them. By binding to the receptor, the protein will either activate it or alternatively block/inhibit it (not allowing the biological process to be initiated).
The activators are called agonists, while the blockers are antagonists.

Agonist vs antagonist. Source: Psychonautwiki
Now, terazosin is an antagonist of the α1-adrenergic receptor, meaning that it specifically targets and blocks the α1-adrenergic receptor. And yes, I know what your next question is going to be:
What is the α1-adrenergic receptor?
The α1-adrenergic receptor is a receptor for the neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). There are 3 different subtypes of α1-adrenergic receptor: α1A-, α1B-, and α1D– (don’t ask me what happen to α1C).
The α1-adrenergic receptors are primarily involved with smooth muscle contraction. By binding to and blocking α1-adrenergic receptors, terazosin reduces peripheral vascular resistance (meaning, it lowers blood pressure).
But recently, the researchers behind this new study discovered that Terazosin has another action in the body: it activates phosphoglycerate kinase 1.
What is phosphoglycerate kinase 1?
Phosphoglycerate kinase 1 is an enzyme that catalyzes the formation of ATP.
What does that mean? What is ATP?
Ok, so the fact that you are reading this, means that your body is producing a lot of ATP. It is the fuel for your cells. It attaches to various proteins and ‘powers’ their function.
You may remember from high school biology class that there are tiny bean-shaped objects within the cells of your body called mitochondria. They are the power house of each cell. They help to keep the lights on. Without them, the party is over and the cell dies.
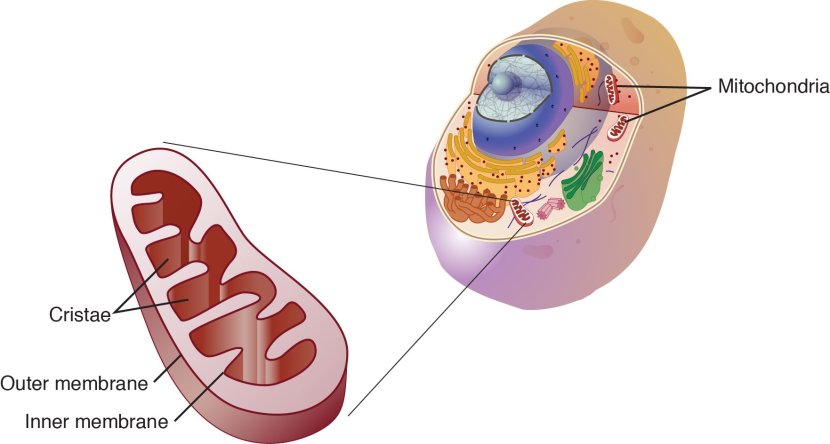
Mitochondria and their location in the cell. Source: NCBI
Mitochondria are very efficient at converting nutrients from food into Adenosine Triphosphate (or ATP). And we need HUGE amounts of ATP in order to do everything we do at any moment of the day.
FUN FACT: The average human body produces/recycles its own weight in ATP every day!
The production of ATP is a multi-step cycle and one of the first actors in that process is Phosphoglycerate kinase 1 (in a step called glycolysis).
And recently the researchers behind the research being reviewed in this post today published this report:
 Title: Terazosin activates Pgk1 and Hsp90 to promote stress resistance.
Title: Terazosin activates Pgk1 and Hsp90 to promote stress resistance.
Authors: Chen X, Zhao C, Li X, Wang T, Li Y, Cao C, Ding Y, Dong M, Finci L, Wang JH, Li X, Liu L.
Journal: Nat Chem Biol. 2015 Jan;11(1):19-25.
PMID: 25383758 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers found that terazosin binds to and activates the enzymatic activity of Phosphoglycerate kinase 1. Interestingly, this activation led to enhanced activity of the heat shock protein/chaperone Hsp90, which plays an important role in cellular homeostasis and promotes multi-stress resistance responses when a cell is damaged or under stress. In cell cultures, they found that terazosin enhanced Phosphoglycerate kinase 1 activity (increasing ATP levels), which blocked cell death when the cells were exposed to toxins.
This result suggested to the investigators that terazosin may have neuroprotective properties, and they wanted to test this idea in models of Parkinson’s.
The researchers started their studies by examining what terazosin does in the brains of mice. They found that terazosin increased the levels of pyruvate – which is the product of glycolysis – and ATP in various regions of the brain, including the substantia nigra (which is a region of the brain severely affected in Parkinson’s).
This result suggested not only that terazosin enters the brain, but also increases ATP production.
 Source: Pinterest
Source: Pinterest
Next the researchers asked if terazosin treatment could protect mice from the neurotoxin MPTP, which kills dopamine neurons in the substantia nigra and induces a Parkinson’s-like state in the animals. They reported that 7 days after the administration of MPTP to the mice, pyruvate and ATP levels dropped significantly, but terazosin treatment prevented this reduction. The drug also reduced the loss of dopamine neurons – even when the researchers delayed initiation of treatment until 7 days post MPTP delivery (a rather remarkable effect!).
They next tested terazosin in two additional neurotoxin models of Parkinson’s (6-OHDA in rats and rotenone in flies), and the drug was found to be neuroprotective across the models. By reducing levels of Phosphoglycerate kinase 1 in flies, the researchers found that the neuroprotective effect of terazosin was lost. This last experiment suggests that the beneficial effects of the drug were coming from Phosphoglycerate kinase 1, rather than the blocking of α1-adrenergic receptors.
Source: Ecolab
Next the scientists tested terazosin in genetic models of Parkinson’s. About 10-15% of cases of Parkinson’s are associated with a genetic variation in regions of DNA that increases the risk of developing the condition. One of these regions of DNA is referred to as PINK1. Errors in PINK1 can result in an early onset (around the age of 30 years old) form of Parkinson’s.
Flies with PINK1 mutations exhibited wing posture defects and decreased ATP levels in the brain. The researchers reported that treating PINK1 flies with terazosin partially reversed these abnormalities.
And the investigators didn’t stop there (seriously, this was a “kitchen sink” report, these guys tested everything!). They also tested terazosin on flies with LRRK2 genetic mutations. Leucine-rich repeat kinase 2 (or LRRK2 – pronounced ‘lark 2’) – also known as ‘Dardarin‘ (from the Basque word “dardara” which means “trembling”) is another region of DNA (or gene) where variations have been associated with increased risk of developing Parkinson’s (Click here to read a previous SoPD post about LRRK2). Flies with mutated LRRK2 genes have motor problems, and guess what? When the researchers tested terazosin on flies with LRRK2 genetic mutations, they observed a partial improvement in those motor problems (it’s becoming a bit repetitive, yeah?).
The researchers next tested terazosin on a genetically engineered mouse that produced very high levels of human alpha synuclein protein, and they reported that the terazosin-treated mice had less alpha synuclein in their brains.
 Neurons growing in petri dishes. Source: Salk
Neurons growing in petri dishes. Source: Salk
And finally, they tested terazosin on human cells in culture. These cells had been collected from people with LRRK2-associated Parkinson’s, and converted into neurons. As the neurons grew in culture, approximately 60% of the LRRK2-mutated neurons started accumulating alpha synuclein protein compared with just 15% of the neurons from healthy individuals. And (you guessed it) terazosin reduced the percentage of LRRK2-mutated neurons with accumulated alpha synuclein.
Basically, every PD model that the researchers tested terazosin on suggested the drug has beneficial effects.
It all sounds almost too good to be true, right?…
…but here’s the thing:
Given that terazosin is a commonly used drug, and has been in clinical use for a long time now (it was patented in 1975 and came into medical use in 1985), the researchers decided to analyse a large medical database to determine if taking this drug reduced the incidence of Parkinson’s.
Using the IBM Watson/Truven database, the investigators found that terazosin (or similar drugs) treatment reduced the risk of having any of the 79 Parkinson’s-related diagnostic codes in your medical file by 20% – relative risk = 0.78 (95% CI: 0.74–0.82).
Whatsmore, when they looked at the Michael J Fox Foundation Parkinson’s Progression Markers Initiative database, the researchers found that individuals with Parkinson’s being treatd with terazosin had a reduced rate of progression on the motor symptoms (although it has to be said here that the number of individuals involved in this analysis was very small).
Either way, the result is very intriguing and reminiscent of the beta blocker/beta agonist report in 2017 (Click here to read a SoPD post about that research).
The report does not explain how enhanced Phosphoglycerate kinase 1 activation could be slowing neurodegeneration or progression in Parkinson’s, but it may open up a new neuroprotective mechansim for future PD research to explore.
Has this neuroprotective effect of Terazosin ever been reported before?
Actually, it sort of has.
Back in 2000, this report was published:
 Title: Overexpression of the alpha1B-adrenergic receptor causes apoptotic neurodegeneration: multiple system atrophy.
Title: Overexpression of the alpha1B-adrenergic receptor causes apoptotic neurodegeneration: multiple system atrophy.
Authors: Zuscik MJ, Sands S, Ross SA, Waugh DJ, Gaivin RJ, Morilak D, Perez DM.
Journal: Nat Med. 2000 Dec;6(12):1388-94.
PMID: 11100125
In this study, the researchers genetically engineered mice that produce high levels of α1B-adrenergic receptor, which developed Parkinson’s-like hindlimb motor problems and displayed neurodegeneration that increased with age.
The investigators found that terazosin partially rescued these mice.
And this initial study was followed up by this report:
Title: Mice expressing the alpha(1B)-adrenergic receptor induces a synucleinopathy with excessive tyrosine nitration but decreased phosphorylation.
Authors: Papay R, Zuscik MJ, Ross SA, Yun J, McCune DF, Gonzalez-Cabrera P, Gaivin R, Drazba J, Perez DM.
Journal: J Neurochem. 2002 Nov;83(3):623-34.
PMID: 12390524 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers further investigated their genetically engineered mice that produce high levels of α1B-adrenergic receptor, and they found that these mice accumulate alpha synuclein over time in brain cells. Interestingly, long‐term treatment with terazosin, not only resulted in protection against the behaviour symptoms and neurodegeneration (as in the first study), but also reduced the accumulation of alpha synuclein.
Wow! Where can I get me some of this terazosin stuff?
Before anyone thinks of doing anything silly, we need to remember that this research needs to be independently validated – both the lab work and the big database analysis. The later should be pretty quick to do (some of the Scandinavian medical databases may be able to help with that task), but the lab work may take some time.
There are also some safety concerns regarding this drug (yes, here comes my “raining on the parade” piece). People with Parkinson’s can have blood pressure issues (Click here to read a previous SoPD post about this), and terazosin is a drug that is used to treat high blood pressure. Thus caution must be taken as we move foward with further investigations of this drug. Anyone with angina (chest pain caused by reduced blood flow to the heart muscles) or low blood pressure should not take this drug.
When the research community shares information like these new research results, they do so knowing that they can’t stop people from trying the drug. But it should always be said that if folks are going to try something experimental, they should discuss it with their doctor first before doing anything. We do not know what this drug might do in people with Parkinson’s at different doses.
I see. So what now? We wait for a clinical trial?
No waiting.
A clinical trial is already funded and about to start.
Que?
The investigators behind the research described above have already received funding to conduct a clinical trial of terazosin in Parkinson’s. The TZ-PD study (I’ll let you work out that acronym) will recruit 20 people with Parkinson’s (Hoehn-Yahr Stage I-III) and randomly assign them 1:1 to either terazosin or placebo treatment for 12 weeks.
It is a double-blind Phase II trial to evaluate the safety and tolerability of 5 milligrams per day of terazosin in people with Parkinson’s. The study is expected to report in January 2020 (Click here to read more about this).
This is a very short study (just 12 weeks treatment), so it will be difficult to determine any disease modifying effects. But the goal here is to see if the drug is actually safe in Parkinson’s. Blood pressure measures are part of the primary measures being used so the investigators are obviously weary about the effect that this hypertension medication could have in a population that can have low blood pressure issues.
It will be interesting to see the results in January and what happens after that.
So what does it all mean?
I have said it before, but I will say it again: The pace of Parkinson’s research news is rather breath taking. I appreciate that this does not make living with the condition any easier, but one has to admit that it feels like a lot of research is being generated at the moment. Last week, Dutch researchers had a positive result in an exercycling clinical trial for Parkinson’s (Click here to read the SoPD post on that), and this week researchers are publishing compelling data supporting the re-purposing of a prostate drug to Parkinson’s. For those of us trying to read/write about it, just keeping up is becoming a full time job!
It is easy to get over excited by it all as well. But as we discussed above, we need to see independent replication of the results before getting carried away.
 Source: Medicinehow
Source: Medicinehow
These new results are interesting and encouraging as they potentially offer us yet another clinically available drug that could be re-purposed for Parkinson’s. And this drug has been around for a long time and is well used, so we have a very clear clinical profile for it. As with all of the drugs being re-purposed by groups like the Cure Parkinson’s Trust (for example, Exenatide, Ambroxol, UDCA, etc) and Michael J Fox Foundation (Nilotinib, Isradipine, etc – the Fox foundation has a nice write up on repurposing of drugs – click here to read it), I am intrigued to see how terazosin will do in the clinical trial currently being conducted.
And expect another SoPD post on this topic when the results of the TZ-PD trial are published.
The banner for today’s post was sourced from





Interest in following terazosin trial in January 2020
LikeLike
Thank you for the quick, easy-access, (yet not too simplistic) description. As ever, hope, but no expectations… 🙂
LikeLike
Hello Martin
First of all my deep appreciation for putting and updating this information, here is a concern I have, since the beginning of the year I had been having numbness and weakness in both legs, a month later I started with lots of constipation and dizziness, sleep disturbances, anxiety, depression and pain in back and a couple of weeks ago definitively both legs are more stiff and making me walk in a funny way, I already visited a couple of neurologist in Mexico since I am living in Sacramento CA and travel to Mexico border is around 8 hrs driving the Neuros in Mexico thinks that this is nothing abnormal, also I visited a neurologist in Sac this week she said the same, only ordered some blood test that I will do again after a had this completed same test in February.
My father has Parkinson since he was 38 years old I am very concerned about this could potentially be the symptoms, although the neurons checked the typical test like toe, arm, finger to nose, etc test they said I am well, this is not PD, how can then explain my stiffness that does not allow me to walk properly? I am trapped in the health system, the drs thinks that all is in my head, that I might need to see a different doctor, the question here is based on your experience what could this be? thanks again
LikeLike
Well, I guess I should thank my enlarged prostate for my long history with Alfuzosin, and my gallstones for Ursodiol.
LikeLike
Thanks as always Simon.
LikeLike
Simon,
Are there any updates on Ambroxol and UDCA you mentioned here? Also could you comment on availability of the blood tests to check glucocerebrosidase activity and LRRK2 protein level for LRRK2 G2019s and GBA N370S heterozygous mutations? I understand these tests are done in research studies but are they available commercially?
Thanks,
Felix
LikeLike
Simon,
Please allow me to share this for mutual learning.
I am 2.5 years past diagnosis (probably the Benign Tremulous PD). My health has improved post diagnosis likely due to exercise. I persuaded my GP to change my Prostate drug from Finasteride to Terazosin 5mg. Blood pressure dropped noticeably and I bought a standard device. My Standing blood pressure was occasionally below 90 Systolic – the level Alskog indicates ‘not good’. I felt occasionally light headed but ever fainted. Forgot to take it one night but took it at 04:00. Felt light headed and after breakfast measured standing at 72/47. I had nicotine patches from a failed experiment so put on 14mg. An hour later my standing pressure was 95/62. I went to my morning Yoga class and participated fully, with slight light headedness. Post Yoga 104/65. Another solution for low PB Alskog suggests is increased salt or salt tablets (but with serious caveats).
I appreciate your work.
LikeLike
Thanks for sharing Dave.
Simon
LikeLike
Hello,
Can you please provide an update on the last testing of Terazosin?
Thanks!
LikeLike
Would like to hear about the test results.
LikeLike
Hi Matt,
Thanks for your comment. I have communicated with the co-ordinators of the study. COVID delayed things, but they will be publishing the results of the study soon. In addition, they are furthering their research into terazosin in a follow up study (funded by the Michael J Fox Foundation) looking at better measures of target engagement (https://clinicaltrials.gov/ct2/show/NCT04551040).
Kind regards,
Simon
LikeLike
Thank you so much for your reply and I’m sure there are many like me looking forward to hearing the results.
LikeLike