|
Stanford University researchers have recently published an interesting report in which they not only propose a novel biomarker for Parkinson’s, but also provide some compelling data for a novel therapeutic approach. Their research focuses on a protein called Miro, which is involved in the removal of old or faulty mitochondria. Mitochondria are the power stations of each cells, providing cells with the energy they require to do what they do. Specifically, the researchers found that Miro refuses to let go of mitochndria in people with Parkinson’s (which could act as a biomarker for the condition). They also found that pharmacologically forcing Miro to let go, resulted in neuroprotective benefits in models of Parkinson’s In today’s post, we will discuss what Miro is, what the results of the new research suggest, and we will consider what will happen next.
|
Source: Amazingaccelerators
Every now and then a research report comes along and you think: “Whoa, that’s amazing!”
It a piece of work that breaks down your cynicism (which you have proudly built up over years of failed experiments) and disciplined scepticism (a critical ingredient for a career in scientific research – mantra: ‘question everything’). And for a moment you are taken in by the remarkable beauty of not just good research, but biology itself.
A couple of weeks ago, one such research report was published.
This is it here:
 Title: Miro1 Marks Parkinson’s Disease Subset and Miro1 Reducer Rescues Neuron Loss in Parkinson’s Models.
Title: Miro1 Marks Parkinson’s Disease Subset and Miro1 Reducer Rescues Neuron Loss in Parkinson’s Models.
Authors: Hsieh CH, Li L, Vanhauwaert R, Nguyen KT, Davis MD, Bu G, Wszolek ZK, Wang X.
Journal: Cell Metab. 2019 Sep 23. [Epub ahead of print]
PMID: 31564441
It’s a really interesting study for several reasons.
So what did they report?
In this study, the researchers were interested in identifying biomarkers for Parkinson’s, and they focused their attention on tiny structures in cells called mitochondria.
What are mitochondria?
Mitochondria are the power stations of each cell, as they provide the cell with the energy that the cell needs to function. They help to keep the lights on.
Without them, the party is over and the cell dies.
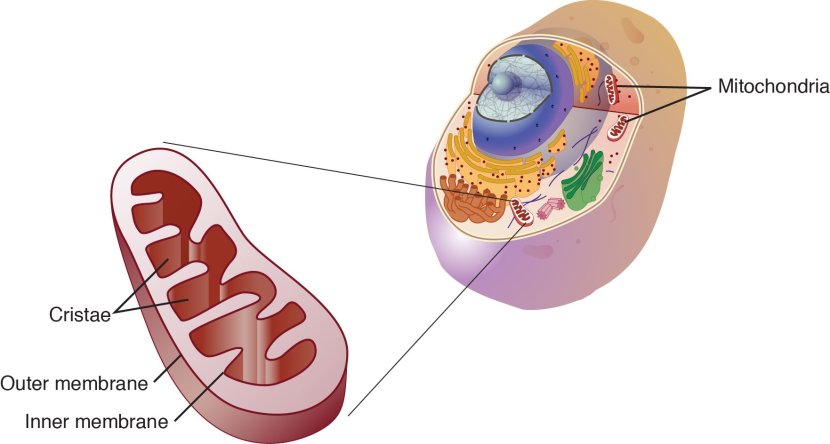
Mitochondria and their location in the cell. Source: NCBI
The image above nicely displays a basic schematic of a mitochondrion (singular), and where mitochondria (plural) reside inside a cell. There are a lot of these bean shaped sources of energy in each cell and they are critical to life as we know it.
Ok, but why were the researchers focusing on mitochondria? What do they have to do with Parkinson’s?
Well, like you, me and all other things in the universe, mitochondria each have a use-by date.
With all their busy power production work, mitochondria get old and worn out (or damaged) with time. When mitochondria becomes sick and needs to be disposed of, they will begin to exhibit mitochondrial stress – the release of messenger molecules that state very clearly “these mitochondria need to be disposed of”. I say ‘very clearly’ because if left unattended, these mitochondria and their messenger molecules can make the cell very sick and ultimately kill it.
As a result, mother nature has devised different mechanisms for disposing of mitochondria. We refer to the process removing sick/damaged mitochondria as mitophagy (a blending of the words mitochondria and autophagy). It involves the mitochondria being engulfed by a structure called a phagophore which is then infused with digestive enzymes from a lysosome, which help to break down the mitochondria.
 The process of mitophagy. Source: Circres
The process of mitophagy. Source: Circres
Are problems with mitophagy associated with Parkinson’s?
Approximately 20% of Parkinson’s cases are associated with particular variations in DNA that render people vulnerable to developing the condition (Click here to read more on the genetics of Parkinson’s). Some of these variations are in specific regions of DNA (called genes) that provide the instructions for making particular proteins.
There are currently 23 genes that are referred to as ‘PARK genes’, as genetic variations within those genes put people at more risk of developing Parkinson’s.
Interestingly, many of these PARK genes provide the instructions for proteins that have various functions in the process of mitophagy.
Two of those proteins are PTEN-induced putative kinase 1 (or PINK1) and PARKIN.
What do PINK1 and PARKIN do?
Both proteins appear to have many different functions, but their roles in the process of mitophagy have been the most studied and are relatively well understood.
PINK1 acts like a kind of handle on the surface of mitochondria. In normal, healthy cells, the PINK1 protein attaches to the surface of mitochondria and it is slowly absorbed until it completely disappears from the surface and is degraded. In unhealthy cells, however, this process is inhibited and PINK1 starts to accumulate on the outer surface of the mitochondria. Lots of handles poking out of the surface of the mitochondria.
Now, if PINK1 is a handle, then PARKIN is a flag that likes to hold onto the PINK1 handle. While exposed on the surface of mitochondria PINK1 starts grabbing the PARKIN protein. This pairing is a signal to the cell that this particular mitochondrion (singular) is not healthy and needs to be removed. The pairing start the process that leads to mitophagy.

PINK1 and PARKIN in normal (right) and unhealthy (left) situations. Source: Hindawi
In the absence of normal PINK1 or PARKIN proteins, there is no handle-flag system and the mitophagy process becomes slower/disrupted. Old/damaged mitochondria can start to pile up and exhibit mitochondrial stress. If they are not disposed of appropriately, the cell gets sick and ultimately dies.
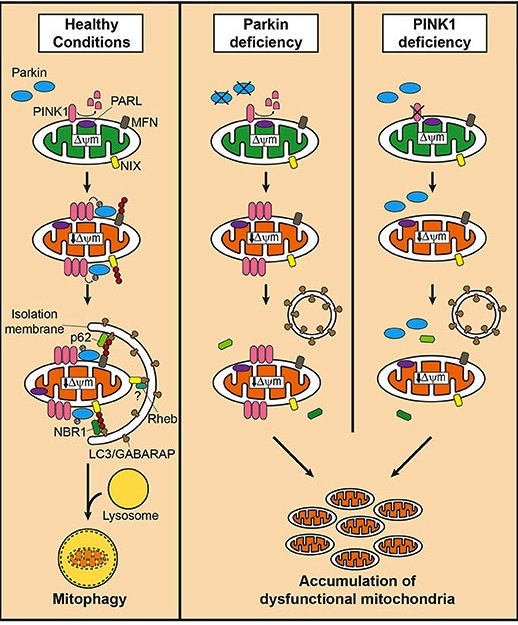 Mitophagy. Source: Frontiersin
Mitophagy. Source: Frontiersin
People with particular genetic mutations in the PINK1 or PARKIN genes are vulnerable to developing an early onset form of Parkinson’s (generally before 40 years of age – again, please note the age). It is believed that the dysfunctional disposal of (and accumulation of) old mitochondria may be part of the reason why these individuals develop the condition at such an early age.
But PINK and Parkin are not only involved with mitophagy, they are also involved with many other functions.
For example, PINK1 has been found interact with components of the motor complex which mediates the transportion of mitochondria around cells (Click here to read more about this).
Mitochondria move around?
Oh yeah. The energy needs of a cell will vary from moment to moment and place to place within the cell, and when the demands are higher in one particular region (for whatever reason), the cell will have mitochondria moved to that region.
To do that, PINK will work with other proteins to help transport the mitochondria.
And one of those other proteins is called Miro.
What is Miro?
Miro is the focus of today’s post.
Mitochondrial Rho GTPase 1 (or MIRO1) is a protein that attaches to the outer surface of mitochondria and interacts with PINK and Parkin:
 Title: PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility.
Title: PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility.
Authors: Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL.
Journal: Cell. 2011 Nov 11;147(4):893-906. doi: 10.1016/j.cell.2011.10.018.
PMID: 22078885 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers reported that Miro is a component of the complex of proteins involved with transporting mitochodria. Specifically, it anchors a protein called kinesin (a key protein in intra cellular transportation of objects) to the mitochondrial surface. The investigators found that Parkinson’s associated PINK and Parkin are responsible for the disposal of Miro. By getting rid of Miro, this action releases kinesin from the surface of mitochondria, which results in the halting of mitochondrial transportation. In this manner, PINK and Parkin are involved with the transportation of mitochondria.
The lead researcher of this report is Assoc. Prof. Xinnan Wang, who runs a research lab at Standford Medical school.
 Dr Xinnan Wang. Source: Zuckermaninstitute
Dr Xinnan Wang. Source: Zuckermaninstitute
Dr Wang’s team next asked whether Miro protein levels and activity are altered in people with Parkinson’s. Given the interaction between two Parkinson’s-associated proteins – PINK and Parkin – they were wondering what actually happens to Miro in people with the condition. Their investigations led to the publication of this report:
 Title: Functional Impairment in Miro Degradation and Mitophagy Is a Shared Feature in Familial and Sporadic Parkinson’s Disease.
Title: Functional Impairment in Miro Degradation and Mitophagy Is a Shared Feature in Familial and Sporadic Parkinson’s Disease.
Authors: Hsieh CH, Shaltouki A, Gonzalez AE, Bettencourt da Cruz A, Burbulla LF, St Lawrence E, Schüle B, Krainc D, Palmer TD, Wang X.
Journal: Cell Stem Cell. 2016 Dec 1;19(6):709-724.
PMID: 27618216 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers collected skin cells (called fibroblasts) from people with and without Parkinson’s. They found that levels of Miro1 protein were normal in the Parkinson’s fibroblasts, when compared to healthy control cells.
When they investigators stressed the mitochondria in the cells, however, they noticed something rather interesting.
Treating the mitochondria to a chemical called carbonyl cyanide m-chlorophenyl hydrazone (let’s just call it CCCP to keep things simple) for 6 hours causes Miro1 to be released and degraded in cells from healthy individuals. But, and here’s where this story starts to get interesting: when the researchers treated fibroblasts collected from 16 patients with Parkinson’s, they found that there was very little detaching and degrading of Miro. Miro was holding on tightly to the surface of the mitochondria.
And the story got even more interesting when the investigators noticed that Miro removal from mitochondria is delayed in cells collected from people with Parkinson’s who have a genetic variation in a third Parkinson’s-associated gene: LRRK2.
What is LRRK2?
Leucine-rich repeat kinase 2 (or LRRK2 – pronounced ‘lark 2’) – also known as ‘Dardarin‘ (from the Basque word “dardara” which means “trembling”) – is an enzyme that has many functions within a cell – from supporting efforts to move things around inside the cell to helping to keep the power on (involved with mitochondrial function).
Genetic variations within the LRRK2 gene are recognised as being some of the most common with regards to increasing ones risk of developing Parkinson’s (LRRK2 variants are present in approximately 1-2% of all cases of Parkinson’s).

The structure of Lrrk2 and where various mutations lie. Source: Intech
As the image above suggests, mutations in the PARK8 gene are also associated with Crohn’s disease (Click here and here for more on this) – though that mutation is in a different location to those associated with Parkinson’s. And one particularly common Parkinson’s-associated LRRK2 mutation – called G2019S – is also associated with increased risk of certain types of cancer, especially for hormone-related cancer and breast cancer in women – Click here to read more about this. If you have a G2019S mutation, no reason to panic – but it is good to be aware of this association and have regular check ups.
Wow. So Miro removal from mitochondria is delayed in Parkin, PINK and LRRK2-associated Parkinson’s?
Dr Wang and her team found that normal LRRK2 protein interacts with Miro, and promotes the removal of Miro from the surface of mitochondria. But they observed that the Parkinson’s associated G2019S-LRRK2 form of the protein delayed the removal of Miro. This delay consequently slowed the initiation of mitophagy.
Next, the researchers wanted to see what kind of affect reducing levels of Miro protein might have in cells with the G2019S-LRRK2 form of protein. Remarkably, partial reduction of Miro protein levels in G2019S-LRRK2 human neuron and fly (Drosophila) models of Parkinson’s restored mitophagy and protected cells from mitochondrial stress. It even rescued the motor issues that are observed in the fly models.
The researchers concluded their study suggesting that the LRRK2 and the PINK1/Parkin pathways function in parallel and converge on interation and degradation of Miro, which led them to question whether “prolonged retention of Miro, and the downstream consequences that ensue, may constitute a central component of PD pathogenesis“.
Could Miro be some kind of central nexus that could be responsible for many cases of Parkinson’s?!?
But hang on a second, you suggested above that very few people with Parkinson’s have a PINK, Parkin or LRRK2 genetic variation. So how do the researchers explain this effect in everyone else with Parkinson’s?
That is a great question.
And about this time last year, Dr Wang and her team published a report providing one possible explanation. This is the report here:
 Title: Alpha-synuclein delays mitophagy and targeting Miro rescues neuron loss in Parkinson’s models.
Title: Alpha-synuclein delays mitophagy and targeting Miro rescues neuron loss in Parkinson’s models.
Authors: Shaltouki A, Hsieh CH, Kim MJ, Wang X.
Journal: Acta Neuropathol. 2018 Oct;136(4):607-620.
PMID: 29923074 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers reported that the build up of Parkinson’s-associated alpha synuclein protein was correlated with increased levels of Miro protein. That is to say, the more alpha synuclein protein that was present in a post mortem brain from a person who passed away with Parkinson’s, the higher the level of Miro protein also detected in that brain. And this finding was replicated in cells which were genetically engineered to produce high levels of alpha synuclein protein (Click here to read more about alpha synuclein and its association with Parkinson’s).
And again, the investigators found that a partial reduction of Miro protein levels in cells with high levels of alpha synuclein rescued the cells. They also repeated this results in a fly model of alpha synuclein-associated Parkinson’s and found that it rescued them as well.
Importantly, the researchers demonstrated that the impact of alpha synuclein on Miro is independent of LRRK2, PINK or Parkin. And these results led the researchers to conclude that their data “positions mitochondria at the center of PD pathogenesis with Miro as a potentially straightforward therapeutic target”.
Has anyone developed any treatments tartgeting Miro?
So this is where we come to the report that was published most recently by Dr Wang and her team. It was the report mentioned at the top of this post:
 Title: Miro1 Marks Parkinson’s Disease Subset and Miro1 Reducer Rescues Neuron Loss in Parkinson’s Models.
Title: Miro1 Marks Parkinson’s Disease Subset and Miro1 Reducer Rescues Neuron Loss in Parkinson’s Models.
Authors: Hsieh CH, Li L, Vanhauwaert R, Nguyen KT, Davis MD, Bu G, Wszolek ZK, Wang X.
Journal: Cell Metab. 2019 Sep 23. [Epub ahead of print]
PMID: 31564441
In this study, Dr Wang and her team wanted to determine in Miro was a useful biomarker for Parkinson’s, and to conduct a drug screen for molecules that can reduce levels of Miro.
They began by collecting skin cells (fibroblasts) from a total of 71 people with Parkinson’s as well as 3 people people who have symptoms that put them at-risk of developing the condition. They also took a large collection of fibroblast cells from healthy control individuals.
The researchers put all of these cells in culture and treated the cells with CCCP.
After 6 hours of CCCP treatment, Miro was removed from the surface of mitochondria in all of the control samples. But after 6 hours of CCCP treatment, the researchers found that Miro was still attached to the mitochondria in 69 of the Parkinson’s and at-risk cells.
For those of you trying to do the maths, that’s 93% of the cases!
That’s amazing! So Miro is a Parkinson’s biomarker?
That would be a happy ending to this story wouldn’t it.
The inability to detach Miro was significantly associated with Parkinson’s, but it was not correlated with disease progression (based on years since diagnosis) or the clinical features of the biopsied patient (according to the UPDRS clinical rating scale). Nor was it associated with age or gender.
So it is a marker of Parkinson’s perhaps, but not any specific aspect of the condition?
Exactly.
What about other neurodegenerative conditions? Is Miro associated with them?
A really interesting question… with an even more interesting answer.
The researchers obtained cells from patients diagnosed with various movement disorders very similar to Parkinson’s, such as:
- Dementia with Lewy Bodies (DLB)
- Frontotemporal Degeneration (FTD)
- Sporadic Corticobasal Degeneration (CBD)
- Sporadic Progressive Supranuclear Palsy (PSP)
And guess what?
Drum roll please…
Miro was effectively degraded by CCCP treatment in all of these cells. The delay in Miro degradation appears to be specific to Parkinson’s.
So Miro sticking to mitochondria is really a biomarker of Parkinson’s?
Exactly.
Ok, so summing up. What does it all mean?
No wait, we haven’t got to the really interesting part yet.
You mean there’s more?
A lot more!
The researchers screened the ability of 6,835,320 commercialized small molecules (that is a lot) for their ability to remove Miro from the mitochondria of Parkinson’s cells. Critically, the investigators filtered the drugs for their ability to be taken orally and for their blood-brain barrier penetrance properties (the blood-brain barrier is a protective membrane surrounding the brain, stopping most drugs from entering).
And guess what?
They found 11 molecules that could reduce Miro in the context of Parkinson’s cells, and they selected one (#3) which they named ‘Miro reducer’ and then tested that molecule on both cells from people with Parkinson’s as well as fly models of Parkinson’s.
The researchers found that ‘Miro reducer’ promoted the clearance of damaged mitochondria and also protected Parkinson’s patient-derived neurons grown in cell culture against mitochondrial stress. In addition, ‘Miro reducer’ rescued fly models of LRRK2-, PINK-, and alpha synuclein-associated Parkinson’s.
WOW! Where can I get me some of that ‘Miro reducer’ stuff?
So Dr Wang has formed a company – called “CuraX” – with the goal of further developing a ‘Miro reducer’ compound.
Dr Wang says “Our hope is that if this compound or a similar one proves nontoxic and efficacious and we can give it, like a statin drug, to people who’ve tested positive for the Miro-removal defect but don’t yet have Parkinson’s symptoms, they’ll never get it.” (Source).
I am also assuming that they will be exploring the possibility of using Miro as a biomarker for Parkinson’s in some kind of diagnostic test.
Sounds amazing. Almost too good to be true?
We will have to wait and see. It will be important to see independent replication/validation of the research.
But it is a fascinating story thus far.
Going forward will not be easy (yes, here comes my “rain-on-the-parade” segment). Manipulating levels of Miro is a delicate business. Complete loss of Miro in genetically engineered mice results in rapidly progressive neurological problems, which mirrors the human condition upper motor neuron syndrome. These problems stem from the inability of mitochondria to be transported around inside of cells (Click here to read more about this research).
And Miro deficiency has been reported in patients with amyotrophic lateral sclerosis (ALS or Motor Neurone Disease – Click here to read more about this research). Interestingy the mutant version of the ALS-associated mutant SOD1 protein appears to reduce the level of Miro in cells (Click here to read more about this research).
It almost begs the question: Are Parkinson’s and ALS two ends of a Miro spectrum?
Either way, dosing of any ‘Miro reducer’ drug for Parkinson’s will be important. And to their credit, Dr Wang and her team address this matter in the discussion of their most recent report.
So what does it all mean?
Mitochondria (the power stations of cells) have long been a focus of Parkinson’s research, and there are a number of clinical trials attempting to improve the function of these important structures (Click here to read a previous SoPD posts on one such clinical trial).
 Cells in culture (blue=nucleus, gold=mitochondria). Source: nigms
Cells in culture (blue=nucleus, gold=mitochondria). Source: nigms
New research from scientists at Stanford University suggests that a protein present on the surface of mitochondria may be not only involved in the pathologenesis of Parkinson’s, but could also be a useful biomarker for the condition – one which could be targeted therapeutically. Their research – if independently verified – could represent a very exciting new area of Parkinson’s research.
The SoPD has not been able to learn anything about the “CuraX” biotech company that has been set up to further develop this research further towards clinical utility, but rest assured I’ll let you know when I hear anything.
I will be watching this space closely.
The banner for today’s post was sourced from genengnews


Thank You
LikeLike
You are very welcome!
LikeLike
Yes thank you. So interesting. It sounds like this would mainly be useful as a biomarker and as a preventive therapy, but not as a therapy for people who already have the disease…
LikeLike
Hi Diana,
Thanks for your comment – glad you liked the post. It is still very early days with this research, but I would guess that it could potentially have beneficial effects for folks already diagnosed with PD – helping to slow down the progression of the condition. Unfortunately we will have to wait and see.
Kind regards,
Simon
LikeLiked by 1 person
Miro has canonical EF hands that indicate it plays a role in sensing cytoplasmic calcium signals. Manipulating its activity pharmacologically may well be tricky as you say., it may well disrupt mitochondrial calcium handling in otherwise healthy cells. Indeed the mito Ca uniporter binds miro-1 (https://www.ncbi.nlm.nih.gov/pubmed/29686046).
The resistance of PD fibroblasts to uncoupler-induced miro-reduction is impressive at 93% especially as skin fibroblasts are not notably disrupted in PD. Curious.
LikeLiked by 2 people
Curious indeed. As I wrote in an earlier comments section, if the defect is present in the cells of a skin fibroblast, doesn’t that indicate that the cause of the defect is in the DNA? But if it was in the genes, it would surely have been discovered by now. Could it be in the non-coding sections of the DNA?
Sporadic Parkinson’s disease derived neuronal cells show disease-specific mRNA and small RNA signatures with abundant deregulation of piRNAs (2018):
https://actaneurocomms.biomedcentral.com/articles/10.1186/s40478-018-0561-x
LikeLike
I am thinking along the lines of a circulating agent, conceivably homocysteine from L-DOPA. Were the 7% drug-naive ?
LikeLike
Hi Jeffryn,
Thanks for the comment. I have had a hard time reconciling the ‘fibroblast issue’ as well, and I am still struggling. I lean on the side of genetics though – while PARK genes can explain ~10-20% of PD cases, my suspicion is that there is a long tail of extremely rare variants in PARK gene associated networks that give rise to a vulnerability for developing PD (this theory is still a work in progress, but hopefully you see where I am going). Variants in a network associated with PINK, for example, might slightly reduce the performance of PINK, but the variant is so rare that it won’t be picked up in the large genetic studies. And this reduced performance probably only shows itself when cells are stressed. Such variants probably start to apply on the individual level and will never show themselve on population studies. Still thinking this one through – will let you know if it ever gets any traction – but it is the only way I can explain the fibroblast issue.
Kind regards,
Simon
LikeLike
Hi Peter,
There are a lot of curious details in this report. The 93% number is just staggering, especially when you take into account the LBD, PSP, CBD,etc analyses. Given the diagnostic error rate of ~10-15% for PD diagnosis in clinics, their source of fibroblasts must be really good. As Jeffryn suggests, a full GWAS analysis of each fibroblast used in the study would be interesting (especially for the 7% negative). Lots of food for thought.
Kind regards,
Simon
LikeLike
Hi Simon, Maybe DNA metyhylation or acetylation during iPD progression has imrinted the fibroblasts ? Proteomic study desirable too ? Peter
LikeLike
Appreciate the background, history, context leading to this latest discovery. While we wait for independent verification of these results, I am wondering how this hypothesized bioprocess fits in or explains current Parkinson’s observations. For example, can it help explain how exercise seems to delay Parkinson’s progression? Does it support certain dietary restrictions or recommendations?
LikeLiked by 1 person
Hi Mark,
Thanks for the great comment – glad you liked the post. The short, honest answer is: Your guess is as good as mine. The longer answer (read: assumption) is that there are lots of exercise-induced mitochondrial adaptations (this review discusses some of them: https://www.hindawi.com/journals/omcl/2019/7058350/), and there are molecules that improve mitochondrial performance (TUDCA, Nico Ribo, etc) while other diet-based elements probably put a lot of pressure on mitochondrial function. By improving mitochondrial performance (via good diet and balanced exercise), perhaps the miro issue is resolved. It would be interesting to look at the full list of miro reducers and see if any patterns appear with regards to some of the molecules and their targets identified. But like I said we need more research on all of this, so I lean more towards the short, honest answer.
Kind regards,
Simon
LikeLike
Those are amazing! We saw a flock almost like that in Colorado one summer at a house near Taylor Reservoir (south central). How is packing for the move coming along?
Love,
Susan
LikeLike
More food for thought?
“Not only did we find PD pathology related phenotypes in long-term cultures of [dopamine neurons (DAn)] representing a monogenic form of PD, but also in those from patients of [idiopathic PD (ID-PD)]. This critical point indicates that the cause for the increased susceptibility of ID-PD-derived DAn to undergo degeneration in vitro after long-time culture, albeit complex, is encoded in the genome of ID-PD patients, or at least of those tested in our study. Therefore, intrinsic cell-autonomous factors,rather than environmental influences, appear to be sufficient to trigger neurodegeneration of DAn from PD patients, in a process that requires time to manifest itself, but that can be modelled within the time-frame of in vitro experiments.”
Disease-specific phenotypes in dopamine neurons from human iPSC-based models of genetic and sporadic Parkinson’s disease (2012):
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3403296/
LikeLike
Another very interesting paper (about 3 years later) by some of the same authors.
Aberrant epigenome in iPSC‐derived dopaminergic neurons from Parkinson’s disease patients (2015):
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4693505/
LikeLike
Or, getting back to fibroblasts.
“… our data indicate that basic mechanisms active in neural cells in PD are likely expressed in other non-neuronal cells suggesting a generalized biological defect in PD.”
Parkinson’s disease skin fibroblasts display signature alterations in growth, redox homeostasis, mitochondrial function, and autophagy (2017):
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5770791/
LikeLiked by 1 person
I had been hoping that AlzForum would do a solid article on the “Miro1 defect”. NeurologyToday beat them to the punch!
“There is something, either genetic or epigenetic, that is persisting across generations of fibroblasts. The fact that this defect occurs in fibroblasts, not just neurons, also supports the idea that PD is a whole-body disease, not just a brain disease.”
“’One conceivable explanation is that we are seeing a readout of the polygenic risk that leads to Parkinson’s disease,’ Dr. Standaert said, but that would require deeper genetic analysis than was done in this study.”
https://journals.lww.com/neurotodayonline/Fulltext/2019/11070/Mitochondrial_Protein_Emerges_as_a_Strong.7.aspx
LikeLike
Regarding how skin cells could provide a sign for Parkinson’s: This brings to mind that melanoma is also strongly associated with Parkinson’s.
And it is not just that if you have Parkinson’s you are more likely to get melanoma; it is also the case that if a close relative got Parkinson’s you are *also* more likely to get melanoma. And vice-versa. So, heredity may be involved, then, just as some have speculated above.
I wonder whether the relationship might be a tendency for the body to overproduce melanin? This tendency is present in melanoma, and we know that neuromelanin is prominently concentrated in the substantia nigra, which is named for the presence of melanin in it. We also know that neuromelanin is a significant stimulator of microglia, so that when it emits from damaged neurons, it agitates microglia in the vicinity into the M1 state of activation, which causes them to attack neurons in a manner that damages bystander neurons, feeding a cycle of autoimmmune inflammation.
So is it possible that neuromelanin is also implicated in the inability of neurons to dissolve the Miro that clings to their mitichondria, when those mitochondria are in decline?
I think it is very important to understand exactly *why* the Miro does not dissolve in response to mitochondrial degradation. What is the process by which it dissolves under normal conditions, and what is it about Parkinson’s that interferes with that process?
I can’t help feeling that the rush to form this company CuraX to monetize a drug that can simply dissolve Miro is ill-advised. As you have pointed out, a drug of this kind could interfere with the ability of kinesin to transport perfectly healthy mitochondria to those areas of the neuron that are most in need of energy, with harmful results.
But if we could determine what is *causing* the Miro not to dissolve, then *that* process could be targeted for interference, so that healthy mitochondria would still be transported, while unhealthy mitochondria got their Miro dissolved and thus could be cleaned up via mitophagy.
LikeLike