|
Canadian scientists recently reported that mice with a specific genetic variation – in the Parkinson’s-associated LRRK2 gene – differ in how they are able to deal with bacterial and viral infections. Curiously, mice with the Parkinson’s-associated LRRK2 mutation could handle a bacterial infection better than normal mice, while mice with no LRRK2 protein struggled against the infection. And the researchers found that this effect was most prominent in female mice in particular. And curiously, when the mice are infected with a dangerous virus, female mice with the Parkinson’s-associated LRRK2 mutation fared worse than their male counterparts. In today’s post, we will discuss what LRRK2 is, review the new research, and explore what the sex difference could mean in terms of Parkinson’s.
|
 Autumn colours. Source: Visitsunlimited
Autumn colours. Source: Visitsunlimited
I am a big fan of Autumn.
The colours and the crisp/bracing air. I love the long, slow afternoon strolls and anticipation of the festive season to come.
But most of all I love the license to eat all the good wintery food. After a summer of salads and light food, there is nothing better that entering a warm cottage or pub, and smelling the hearty food (my wife if French – we navigate based on the quality of eateries).
 Autumn bliss. Source: Askdrake
Autumn bliss. Source: Askdrake
But there is a down side to autumn: The start of the flu season.
Luckily, our immune systems are pretty robust – doing battle on a moment-to-moment basis with all manner of pathogenic agents.
Recently, some Canadian scientists discovered something interesing about the immune system and it relates to Parkinson’s.
What did they find?
They found that a certain genetic variation which can increase one’s risk of developing Parkinson’s, may also affect how well one can handle a bacterial or viral infection.
Here is the report of their results:
 Title: Lrrk2 alleles modulate inflammation during microbial infection of mice in a sex-dependent manner
Title: Lrrk2 alleles modulate inflammation during microbial infection of mice in a sex-dependent manner
Authors: Shutinoski B, Hakimi M, Harmsen IE, Lunn M, Rocha J, Lengacher N, Zhou YY, Khan J, Nguyen A, Hake-Volling Q, El-Kodsi D, Li J, Alikashani A, Beauchamp C, Majithia J, Coombs K, Shimshek D, Marcogliese PC, Park DS, Rioux JD, Philpott DJ, Woulfe JM, Hayley S, Sad S, Tomlinson JJ, Brown EG, Schlossmacher MG
Journal: Science Translational Medicine, 25 Sep 2019, 11 (511), eaas9292.
PMID: 31554740
In this study, the researchers were interested in what impact genetic variations in a section of DNA called LRRK2 has on the ability of the immune system to handle bacterial or viral infections.
What is LRRK2?
Leucine-rich repeat kinase 2 (or LRRK2 – pronounced ‘lark 2’) is a gene (a functional section of DNA) that is associated with Parkinson’s. It is also known as PARK8 (Click here to read more about PARK genes and the genetics of Parkinson’s).
Also known as ‘Dardarin‘ (from the Basque word “dardara” which means “trembling”), LRRK2 is an enzyme that has many functions within a cell – from supporting efforts to move things around inside the cell to helping to keep the power on (involved with mitochondrial function).

The many jobs of LRRK2. Source: Researchgate
The LRRK2 gene is made up of many different regions. Each of those regions is involved with the different functions of the eventual protein. As you can see in the image below, the regions of the LRRK2 gene have a variety of different functions:
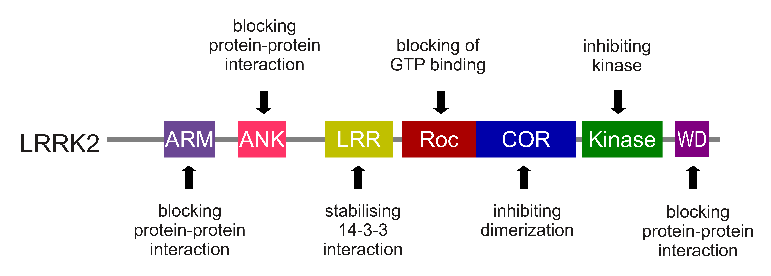
The regions and associated functions of the LRRK2 gene. Source: Intechopen
Genetic errors or variations within the LRRK2 gene are recognised as being some of the most common with regards to increasing ones risk of developing Parkinson’s (as I mentioned in the intro, LRRK2 variants are present in approximately 1-2% of all cases of Parkinson’s).

The structure of Lrrk2 and where various mutations lie. Source: Intech
As the image above suggests, mutations in the PARK8 gene are also associated with Crohn’s disease (Click here and here for more on this) – though that mutation is in a different location to those associated with Parkinson’s. And one particularly common Parkinson’s-associated LRRK2 mutation – called G2019S – is also associated with increased risk of certain types of cancer, especially for hormone-related cancer and breast cancer in women – Click here to read more about this. If you have a G2019S mutation, no reason to panic – but it is good to be aware of this association and have regular check ups.
The G2019S mutation (the name designates its location on the gene) is the most common LRRK2 mutation. In some populations of people it can be found in 40% of people with Parkinson’s (Click here to read more about this). But what is interesting about this mutation is that it gives rise to a LRRK2 enzyme that is hyperactive.
The structure of LRRK2 protein. Source: Wikipedia
As a protein, LRRK2 interacts with many different types of other proteins, and you can imagine that in a finely balanced environment like the cells that a mutant ‘hyperactive’ form of LRRK2 is going to cause problems. The consequences of this constantly active form of LRRK2 protein is believed to be influential in the cell death in LRRK2-associated Parkinson’s.
Thus, biotech companies have been looking for compounds that help to inhibit or modulate LRRK2 activity (Click here to read a previous SoPD post regarding an example of a biotech company – called Denali – which is taking this approach).
Interesting. Why were the scientists interested in effect of LRRK2 on the immune system?
So one of the interesting things about LRRK2 is that levels of this protein in the brain are quite low (particularly in dopamine neurons, which are particularly vulnerable to Parkinson’s).
The highest levels of LRRK2 RNA (which gives rise to LRRK2 protein) are found in the lungs and in granulocytes:
 Source: ProteinAtlas
Source: ProteinAtlas
Lungs I am familiar with. But what are granulocytes?
Granulocytes are a group of white blood cells (leukocytes) that help the immune system fight off infection. There are three types of granulocytes: neutrophil granulocytes, eosinophil granulocytes, and basophil granulocytes:
 Granulocytes. Source: Sinobiological
Granulocytes. Source: Sinobiological
Granulocytes are phagocytes, meaning that they are able to ingest foreign cells such as bacteria, viruses etc. Granulocytes derive their name from the many granules of enzymes they carry, which help to digest the ingested microbes. Granulocytes can account for approximately 60% of our white blood cells.
Given the role of granulocytes in helping the body deal with infections, the researchers behind the new study were curious to see what happens to the immune system when it is affected by different Parkinson’s associated genetic mutations (such as G2019S).
So what did they find?
Firstly they found that in human blood the levels of LRRK2 RNA were highest in neutrophils (a type of granulocyte, mentioned above), followed by monocytes and then B cells. In addition, the researchers looked at human postmortem tissue from conditions associated with inflammation (such as Crohn’s disease and Parkinson’s) and found that LRRK2 was abundant in the leukocytes that were infitrating various tissues, including samples of brain.
And they also reported that in mice, the absence of LRRK2 had no discernible influence on the number of different types of blood cells. So LRRK2 was not required for the production of granulocyte, monocytes or B cells.
So all of this data supports the idea that LRRK2 is present in immune cells.
Next, the investigators took mice with either normal LRRK2 or genetically engineered mice that had no LRRK2 or the Parkinson’s associated G2019S mutation, and they infected them with Salmonella typhimurium, which causes sepsis.
 Salmonella typhimurium. Source: Isglobal
Salmonella typhimurium. Source: Isglobal
What is sepsis?
Sepsis occurs when the immune system overreacts to an infection, and it starts to damage the body’s own organs.
And this is where the findings of the study start to get interesting.
The researchers found that the presence of LRRK2 protein was protective, meaning that mice carrying the normal form of LRRK2 protein could handle the infection while mice without LRRK2 struggled. And curiously, female mice with no LRRK2 managed the infection worse than their male counterparts (that is males with no LRRK2 protein).
On top of this, mice carrying the Parkinson’s associated G2019S mutation controlled infection better than the mice with normal LRRK2. They were able to reduce bacterial growth and survived longer during sepsis. The G2019S-LRRK2 appeared to rev up the immune response.
They repeated the experiment with a reovirus – a type of virus that results in brain inflammation within 3 week of infection in mice.
 Reovirus. Source: Navigator
Reovirus. Source: Navigator
Again, female with no LRRK2 protein fared poorly. They had higher levels of reovirus in the brain than the male mice with no LRRK2 protein, and their survival was very poor compared to the males (who were comparable to normal mice).
Of interest here, however, was the observation that the female mice carrying the Parkinson’s-associated G2019S mutation had worse outcomes than normal mice. Both the male and female G2019S-LRRK2 mice mounted robust immune responses (they had 3x as many leukocytes infiltrating the brain as normal mice, and the viral load was only 10% of the normal mice despite the sex difference), but females with the G2019S mutation did not survive the infection very well (with 3x the risk of dying compared to normal female mice). By comparison, male mice with the G2019S mutation survived as well as normal mice.
Interesting. Has this sex difference ever been observed in humans with LRRK2 genetic variants?
Yes it has.
In general, men are 1.5 times more likely to develop Parkinson’s than women , but this pattern is not observed in people with LRRK2-associated Parkinson’s – women with LRRK2 genetic variants have an equal chance to men of developing the condition (Click here to read more about this).
Another really interesting finding in the study was the observation that the G2019S-LRRK2 mice produced more alpha synuclein when infected by the reovirus. In fact, they produced 50% more alpha synuclein in their brains than the normal mice (and curiously, there was no sex difference here). The researchers behind this study have previously reported anti-viral properties of alpha synuclein (click here to read more about that).
The researchers concluded that “LRRK2 alleles may alter the course of microbial infections by modulating inflammation, and this may be dependent on the sex…“. Their findings focus attention on the potential role of systemic inflammation in Parkinson’s, but how this may relate to the development of Parkinson’s is still to be determined. And the researchers are also keen to evaluate how the effect of LRRK2 inhibition (which as we mentioned above is being developed for the clinic as a possible treatment for Parkinson’s) may affect the immune system response.
So what does it all mean?
Recently Canadian researchers have reported that Parkinson’s-associated LRRK2 genetic variations affect how the immune system responds to both bacterial and viral infections. The G2019S variation appears to boost the immune response, but it does so in a gender-specific manner.
We have previously explored the idea that some of the genetic variations associated with Parkinson’s may actually infer some kind of evolutionary advantage to individuals early in life, but then go on to be detrimental by increasing the risk of Parkinson’s (Click here to read a previous SoPD post on this topic). This new research could be seen in that light. But it is extremely curious how the effect differs between the sexes (in mice at least).
It will be interesting to see if there are any differences between males and females in the currently ongoing LRRK2 inhibition clinical studies. If G2019S-LRRK2 is generating a more robust immune response in females, which may protect them from peripheral infection, but then it backfires in the brain, does this mean that LRRK2 inhibition might be more effective in the ladies (but could potentially leave them more vulnerable to infection)? We are just speculating here and we will wait to see what the results of those clinical trials suggest.
But it is intriguing.
Oh, and this gender difference issue in Parkinson’s is going to explored further in a future SoPD post which will look at research suggesting that a recently negative clinical trial result might only be negative because of the male participants. More on that soon.
The banner for today’s post was sourced from Wikipedia


“… the G2019S-LRRK2 mice produced more alpha synuclein when infected by the reovirus. In fact, they produced 50% more alpha synuclein in their brains than the normal mice …”
As we know from familial PD, too much alpha synuclein, in itself, can cause PD. This seems to provide more support for the idea that when the “mechanism” responsible for clearing (excess) alpha synuclein gets overloaded, the result is PD.
LikeLike
It also provides a possible explanation for why not everyone who has the G2019S mutation ends up getting PD. You might need the G2019S mutation plus a certain number of infections (or a certain length of time with infection).
LikeLike
jeffreyn, I think you are absolutely right. We discuss the model you propose in this open access article:
https://www.ncbi.nlm.nih.gov/pubmed/30342839
LikeLike
Thanks Patrik.
A recent paper from Michael Zasloff’s lab also discusses the possible consequences of too much alpha synuclein.
“Overproduction of alpha synuclein in the enteric nervous system (ENS) and its chronic trafficking to the CNS may damage nerves and lead to Parkinson’s disease.”
Gastrointestinal Immunity and Alpha-Synuclein (September 2019):
https://content.iospress.com/articles/journal-of-parkinsons-disease/jpd191702
LikeLike