|
Things were a bit quiet on the SoPD over the summer, but for good reasons. There was a short hiatus for a family break, but the rest of the time I was rather occupied with the day job. Tremendous efforts were being made at the Cure Parkinson’s Trust, as we were gearing up for our main event of the year: the Linked Clinical Trials (LCT) meeting. This is an annual meeting at which 20 Parkinson’s experts from around the world, gather for a two day face-to-face pow-wow. They evaluate dossiers which contain everything we know about 20+ compounds which have exhibited potential for disease modification in Parkinson’s. The goal of the committee is to decide which of them is ready for clinical evaluation. The writing of those LCT dossiers is a year long exercise, which inevitably becomes a bit of a panic in June and July (hence the lack of activity here at SoPD HQ during that period). It is a mammoth, marathon task, but as you shall see it is one that I rather like. In today’s post, we will discuss what the Linked Clinical Trials initiative is, the process behind the project, and some of the progress being made by the programme.
|
 Archimedes. Source: Lecturesbureau
Archimedes. Source: Lecturesbureau
Archimedes of Syracuse (287 BC – 212 BC) the ancient Greek mathematician, once said that the “shortest distance between two points is a straight line“.
My dad (who is not a regular readers of this blog, but is possibly on par with Archie – just in case he does ever read this) has often been heard saying “Just get to the point Simon“.
 Source: Actioncoach
Source: Actioncoach
Millennia apart, but their collective wisdom is same: Ignore everything else, and get straight to the heart of the matter as quickly as you can.
And this is one of the aspect I really like about the Linked Clinical Trials initiative.
It is all about getting to potentially disease modifying treatments for Parkinson’s to the community as quickly as possible.
What is the Linked Clinical Trials programme?
The LCT initiative was set up 8 years ago by the Cure Parkinson’s Trust with the goal of rapidly repurposing drugs that may have disease modifying potential for Parkinson’s.
What is meant by repurposing?
Drug repurposing (repositioning, reprofiling or re-tasking) is a strategy of identifying novel uses for clinically approved (or experimental) drugs that fall outside the scope of the original medical indication.
An example of this is “Viagra”.
It was riginally developed as an anti-hypertensive medication, and was hugely more successful in the treatment of erectile dysfunction.
The strategy has been adopted and applied by many organisations because it allows for the by-passing of large parts of the drug discovery process, saving time and resources in getting new treatments to the clinic.
 Source: Austinpublishinggroup
Source: Austinpublishinggroup
By repurposing a clinically approved drug – for which we may know a great deal about already in terms of safety, tolerability and dose range – we can skip large parts of the clinical trial process and jump straight to testing the drug in our population of interest (in this case people with Parkinson’s).
In addition to repurposing drugs, the LCT initiative also seeks to catalyse the clinical testing of treatments that may not necessarily be tested by biotech or pharmaceutical companies. Some of these molecules may have existed for a long time, but with no patent protection they have never been clinically tested. And if there is no apparent financial gain to be had, why would anyone test them?
How did the LCT programme start?
The tale begins not with neurology, but cardiology.
Specifically, a cardiologist. His name is Dr Richard Wyse:
 Dr Richard Wyse with an LCT dossier. Source: Trendsmap
Dr Richard Wyse with an LCT dossier. Source: Trendsmap
Richard has been the driving force behind the development and progress of the LCT programme. It is is basically his brain child. And it all began when Richard met the legandary Tom Isaacs (Parkinson’s advocate and one of the co-founders of CPT) at a scientific conference shortly after the Trust was set up. They got talking and over a period of time Tom eventually talked Richard into joining the fledgling organisation.
 The man, the myth, the legend: Tom Isaacs
The man, the myth, the legend: Tom Isaacs
Richard joined the Cure Parkinson’s Trust in 2007 and he immediately began working on preparations for both the Exenatide and GDNF clinical trial programmes for Parkinson’s (Click here and here to read previous SoPD posts about those drugs).
In 2009, Tom was becoming increasingly frustated with the lack of research around the world that was focused on actually “curing” Parkinson’s. He did not want a new iteration on an old symptomatic treatment, he was looking for treatments that would actually change the trajectory of the condition. And in their conversations at CPT HQ, Tom kept asking Richard “What can we do to accelerate things?”
This question resonated with Richard and gradually the seed of an idea came to life. And that idea gave rise to the Linked Clinical Trials initiative.
In essence, the initiative involves a committee of international Parkinson’s experts meeting annually to evaluate a number of compounds for their disease modifying potential in Parkinson’s, and the committee would prioritise which one should be clinically evaluated. Once prioritised CPT would be mandated to getting that drug into a clinical trial for disease modification in Parkinson’s.
Here is a video of Dr Richard Wyse providing an overview of the LCT intiative:
In 2010, the Cure Parkinson’s Trust began building the committee of Parkinson’s experts for the Linked Clinical Trials initiative, and they asked Prof Patrik Brundin who is the director of the Center for Neurodegenerative Science and Jay Van Andel Endowed Chair at the Van Andel Institute (Michigan) to be the chairperson of the LCT committee.
Prof Brundin is a world-renowned scientist in the field of Parkinson’s research. He has more than 30 years of experience (and over 350 research articles) investigating Parkinson’s pathology and experimental therapeutic approaches for the condition. He has also played an instrumental role in the development of the LCT programme.
 Prof Patrik Brundin. Source: Mlive
Prof Patrik Brundin. Source: Mlive
Here is Prof Brundin discussing the LCT initiative:
The Cure Parkinson’s Trust and the Van Andel Institute formed a partnership to take the LCT programme forward, and with tremendous support from both groups there has been no looking back:
 Prof Brundin & Tom Isaacs signing the partnership agreement. Source: CPT
Prof Brundin & Tom Isaacs signing the partnership agreement. Source: CPT
Current members of the LCT committee include some of the best Parkinson’s researchers in the world:
- Prof Roger Barker (University of Cambridge, UK)
- Prof Flint Beal (Cornell University, USA)
- Dr Camille Carroll (Plymouth University, UK)
- Dr Mark Cookson (NIH, USA)
- Prof Ted Dawson (Johns Hopkins University, USA)
- Prof David Devos (University of Lille Nord de France)
- Prof Jeffrey Conn (Vanderbilt University, USA)
- Prof Howard Federoff (University California at Irvine, USA)
- Dr Brian Fiske (The Michael J Fox Foundation)
- Prof Tom Folytnie (University College, London, UK)
- Prof Karl Kieburtz (University of Rochester, USA)
- Prof Dimitri Krainc (Northwestern University Feinberg School of Medicine, USA)
- Prof Andrew Lees (University College London, U.K)
- Prof Mark Mattson (Johns Hopkins University, USA)
- Prof Michael Schwarzschild (Harvard Medical School, USA)
- Prof David Simon (Harvard Medical School, USA)
- Prof David Sulzer (Columbia University, USA)
- Prof Caroline Tanner (University of California, San Francisco, USA)
- Prof John Trojanowski (University of Pennsylvania, USA)
The first LCT meeting was held in 2012, and a report on the initiative and that meeting was published early the next year.
This is the report:
 Title: Linked clinical trials–the development of new clinical learning studies in Parkinson’s disease using screening of multiple prospective new treatments.
Title: Linked clinical trials–the development of new clinical learning studies in Parkinson’s disease using screening of multiple prospective new treatments.
Authors: Brundin P, Barker RA, Conn PJ, Dawson TM, Kieburtz K, Lees AJ, Schwarzschild MA, Tanner CM, Isaacs T, Duffen J, Matthews H, Wyse RK.
Journal: J Parkinsons Dis. 2013 Jan 1;3(3):231-9. doi: 10.3233/JPD-139000. Review.
PMID: 24018336 (This report is OPEN ACCESS if you would like to read it)
In this report, the committee discussed some of the drugs that were evaluated that year. Since that first meeting, however, the committee has decided not to put the results of subsequent meetings in the public domain for two important reasons:
- Confidentiality – some of the information being discussed at the meeting is of a confidential and (in some cases) commercially sensative nature, and
- Safety – very little is known about what might be the right dose to be effective for each compound (if a right dose even exists). And even if an effective dose can be ‘estimated’, even less may be known about the safety of that particular dose. Such safety testing needs to be carried out at hospital research centres.
Thus, the results of the meetings are now not disclosed.
So how are the drugs selected?
Ok, so this is my favourite part of the whole thing.
In fact, the process of identifying drugs/treatments of potential interest for the LCT meetings has very quickly become something of an obsession for yours truly.
And I’m talking “call the divorce lawyers”-kind of obsession.
I can regularly be found sitting in my arm chair at home at 3 am snooping around the internet looking for clues or connections between Parkinson’s and a particular drug. It is like a treasure hunt or a jigsaw puzzle – but one that I am utterly consumed by.
 Source: Medium
Source: Medium
The research team at the Cure Parkinson’s Trust maintains a constant vigil on the neurodegenerative research being published, but we will also often go walking off into left field seeking novel ideas from other medical conditions (such as oncology). We also regularly ask for and discuss new ideas with LCT committee members.
Once we find something interesting, we will approach researchers and talk to biotech companies about compounds that target a particular biological pathway of interest.
Many of these biotech companies are not focused on Parkinson’s or neurodegeneration (or even considering it as a possible indication for their drug). They may have a drug that is being clinically tested for a liver condition, and its disease modifying potential in a brain-related condition is definitely not part of their business plan or even on their radar.
On other occasions (as we discussed above), a particular drug/molecule of interest may not have any intellectual property attached to it and there will be no company to talk to.
Each case is different.
When an interesting drug is identified, the CPT research team will begin investigating everything that is known about the basic characteristics of the drug and what it does in the body. For example, in most cases it is important to know how well:
- the drug can access the brain which is surrounded by a protective membrane that limits most drugs from entering. This is called ‘brain penetrance’.
- the drug is absorbed by the body. This is called ‘bioavailibility’.
- the drug lasts in the body. This is called ‘half-life’.
- the action of the drug can be measured. This is called ‘read out’.
Most critically, the committee will be focused on the safety and tolerability of the compounds being discussed as they do not want to prioritise a treatment that could potentially do harm or reduce the well being/quality of life of the individuals being tested. So drugs that have been tested in humans before and have a well known clinical profile will have an advantage over others.
Once the CPT research team has all of this information, they will then decide to write a dossier for the drug to present to the Linked Clinical Trials committee for evaluation.
What goes into the dossier?
Everything.
Everything that we know about the drug and also the argument/rationale for testing it in Parkinson’s for disease modification. Here is a video of someone taller, but less attractive than Richard showing you what the collection of dossiers looks like:
(Face for radio, voice for silent film)
And what happens at the meeting itself?
So the meeting is a 2 day event held at the Van Andel Institute in Grand Rapids (Michigan).
 The Van Andel Institute. Source: VAI
The Van Andel Institute. Source: VAI
It is a closed door, invite-only meeting, but serious efforts are made to have representatives from all of the major Parkinson’s organisations in the room. For example, the Michael J Fox foundation, the Silverstein Foundation, Parkinson’s UK, and Shake it Up Australia all have representatives at the meeting. Government and regulatory organisations (such as the NIH) are also invited. But critically a group of patient advocates are also in attendance.
Thus, all of the major stakeholders are present.
The LCT committee sit at the front of the room in a horse shoe arrangement, and individuals members will present a dossier for discussion. Each dossier is debated for about 30 minutes. The other attendees in the room are regularly asked for their opinion/thoughts, and there is a very dynamic discussion before the committee members are asked to vote on each dossier.
At the end of the day, the dossiers will be ranked according to their scores and 3-5 drugs will be prioritised for clinical testing.
The job of the LCT committee is primarily drug selection, but given the expertise sitting around the table there is usually some discussion about what potential clinical trial design might look like and which subtypes of Parkinson’s could be investigated for particular drugs.
Interesting. So what has been the outcome of this process?
The Linked Clinical trial programme has given rise to 16 drugs being clinically tested in 17 clinical trials at research centres around the world. One of these clinical trials includes a Phase III study which has just started.
Can you give an example of a drug that was prioritised?
Exenatide (or Byrureon) – the diabetes treatment – was the first drug to be prioritised in the LCT process.
There was preclinical data supporting a neuroprotective effect in models of Parkinson’s and a great deal was known about the clinical profile of the drug. The first clinical trial of Exenatide in Parkinson’s was a phase I trial which was initiated to determine if the drug was safe to use in people with Parkinson’s.
The results of the trial were published in 2013:

Title: Exenatide and the treatment of patients with Parkinson’s disease.
Authors: Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T.
Journal: J Clin Invest. 2013 Jun;123(6):2730-6.
PMID: 23728174 (This study is OPEN ACCESS if you would like to read it)
The researchers gave exenatide (the Byetta formulation which is injected twice per day) to a group of 21 people with moderate Parkinson’s and evaluated their progress over a 14 month period. They compared those participants to 24 additional subjects with Parkinson’s who acted as control (they received no treatment). Exenatide was well tolerated by the participants, although there was some weight loss reported among many of the subjects (one subject could not complete the study due to weight loss).
Importantly, the exenatide-treated subjects demonstrated improvements in their Parkinson’s movement symptoms (as measured by the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (or MDS-UPDRS)), while the control patients continued to decline.
Interestingly, in a two year follow up study – which was conducted 12 months after the subjects stopped receiving exenatide – the researchers found that participants previously exposed to exenatide demonstrated a significant improvement (based on a blind assessment) in their motor features when compared to the control subjects involved in the study.
It is important to remember, however, that this trial was an ‘open-label study’ – that is to say, the participants knew that they were receiving the exenatide treatment so there is the possibility of a placebo effect explaining the improvements. And this necessitated the testing of the efficacy of exenatide in a phase II double blind clinical trial.
And the results of that trial were published last August (2017):
 Title: Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial
Title: Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial
Authors: Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T
Journal: Lancet 2017 Aug 3. pii: S0140-6736(17)31585-4.
PMID: 28781108
In this study, the investigators recruited 62 people with Parkinson’s (average time since diagnosis was approximately 6 years) and they randomly assigned them to one of two groups, exenatide (the Bydureon formulation which is injected once per week) or placebo (32 and 30 people, respectively). The treatment was given for 48 weeks (in addition to their usual medication) and then the participants were followed for another 12-weeks without exenatide (or placebo) in a ‘washout period’.
It is important to remember that in this trial everyone was blind. Both the investigators and the participants. This is referred to as a double-blind clinical trial and is considered the gold standard for testing the efficacy of a new drug.
The researchers found a statistically significant difference in the motor scores of the exenatide-treated group verses the placebo group (p=0·0318). As the placebo group continued to have an increasing (worsening) motor score over time, the exenatide-treated group demonstrated improvements, which remarkably remained after the treatment had been stopped for 3 months (weeks 48-60 on the graph below).
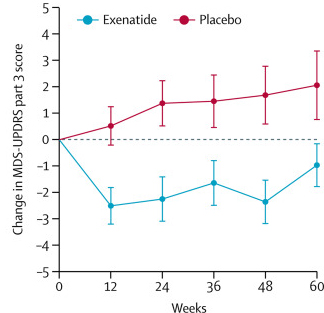
Reduction in motor scores in Exenatide group. Source: Lancet
Brain imaging (DaTscan) also suggested a trend towards reduced rate of decline in the exenatide-treated group when compared with the placebo group. Interestingly, the researchers found no significant differences between the exenatide and placebo groups in scores of cognitive ability or depression – suggesting that the positive effect of exenatide may be specific to the dopamine or motor regions of the brain. It should be noted here that the Phase II clinical trial for Exenatide was also supported by the Michael J Fox Foundation.
A Phase III clinical trial for exenatide has recently been initiated to determine if the drug is having a long term impact on Parkinson’s, or simply a symptomatic effect. There is going to be a SoPD post discussing that trial coming up very shortly.
But in addition to exenatide, there are a LOT of other LCT clinical trials underway.
And at the 2019 LCT meeting, the whole event started with an overview of the ongoing LCT studies, which included news that:
- The Ambroxol trial has finished and the results will be published soon (Click here to learn more about this).
- The EPI589 clinical study has finished and the results will be published soon.
- The Exenatide/Bydureon Phase III trial was shortly starting (Click here to learn more about this).
- The Simvastatin trial in the UK will be finishing next year (2020 – click here to learn more about this).
- The Deferiprone study in France will be finishing next year (2020 – click here to learn more about this).
- The Liraglutide trial in California will be finishing next year (2020 – click here to learn more about this).
- The Lixisenatide Study in France is proceeding according to plan (Click here to learn more about this).
- The Nilotinib trial in the US is proceeding according to plan (Click here to learn more about this).
- The UDCA trial in the UK is underway and recruitment is nearly complete (Click here to learn more about this).
And a lot of other news/updates was shared with the committee members.
Sounds amazing. But why is the meeting only once per year?
The answer to this question is a combination of the availability of the LCT committee members (who are all very busy running major research programmes) and a limited amount of resources (it takes time and money getting clinical trials started).
So what does it all mean?
It doesn’t need to be said, but there is currently a desperate need for novel therapies that will slow, stop or reverse Parkinson’s.
There is a great deal of Parkinson’s preclinical research that is focused on clinically available drugs (for a good example see the recent SoPD post on the prostate drug Terazosin – click here to read that post). We don’t need to re-invent the wheel and come up with new formulations of old drugs to test whether a theory might work. That process requires years of research and longer clinical safety testing. We can use a clinically available drug, which will give us a proof of concept as to whether the hypothesized biology is important/relevant to Parkinson’s.
The Linked Clinical Trials initiative is the fastest way that the Cure Parkinson’s Trust and Van Andel Institute can think of to get novel therapies to the Parkinson’s community. And as we now seek to get more LCT prioritised drugs into clinical trial, I am already getting excited about the 2020 LCT meeting (at present I have 5 compounds of interest and the next meeting is still 10 months away!).
EDITOR’S NOTE – The author of this post is an employee of the Cure Parkinson’s Trust and utterly obsessed with the Linked Clinical Trials process, so he might be a little bit biased in his views on the topic. The trust has not requested the production of this post, but the author considered it interesting and important to share with the Parkinson’s community.
In addition, the information provided by the SoPD website is for educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from CPT





Looks like fun! Great that you include pwp. So impressed by both the concept and the commitment of the brilliant researchers. A toast to CPT and to all who attended. Clink! Clink! Thanks Simon!
LikeLike
Fascinating and inspiring blog. Thanks Simon.
LikeLike
Hi Simon. This is very exciting and I’m so grateful that you are so consumed by solving this puzzle (although I must say I have some sympathy for your family). Is the Australian Parkinson’s Mission represented in LCT? It seems like they have dedicated a huge amount of money to testing drugs for repurposing.
LikeLike
TERAZOSIN not included?
LikeLike
Hi Gustavo,
Thanks for your comment. We probably won’t look at Terazosin as a clinical trial programme is already underway in Iowa (https://clinicaltrials.gov/ct2/show/NCT03905811). We are of course happy to help in any way we can. For more information about Terazosin, see the recent SoPD post on the topic (https://scienceofparkinsons.com/2019/09/18/terazosin/).
Kind regards,
Simon
LikeLike
I find it interesting that this amateur can identify at least five people in that group photo. You clearly have The Who’s who.
Thanks as always Simon.
LikeLike
Hi Double,
We have the best people… no, wait – that came out wrong.
All kidding aside, the LCT committee are the best of the best. And the Parkinson’s advocates are amazing individuals as well. In fact, I am probably the weakest link in the room. Seriously.
Kind regards,
Simon
LikeLike
Hi Simon,
Thank you for another interesting post. Are you able to let us know about the ranking process to determine the shortlist – do you use a formal process to decide (using a weighting for each criteria) or is it based on a straight vote after discussion of the dossier?
Best regards,
Paula
LikeLike
Thank you Simon, great post as always! I think the LCT-program is an amazing idea with enormous potential and I have been following the work with great interest for several years. I think that recent work has clearly demonstrated that there’s one key element lacking for the important work to fully reach fruition, and that is actively working for policy change when it comes to how drugs are moved from being identified as potentially providing an effect for a disease group to actually being available to patients in that group through the healthcare system. In the example of Exenatide, despite the drug being known to be (reasonably) safe for one group, that is partly overlapping, and potentially with an important benefit for PD, it is still not available to PwP in many countries around the world.
LikeLike
I just want to thank you for the work that you do, and for sharing it with all of us in the PD community. My sweet, beloved mom has Parkinson’s and your blog is a ray of hope and optimism. I always feel better after poking around here.
LikeLike
Easy to check out, readable…heck I needed to leave a commment!
LikeLike