|
Recently two independent research groups published scientific papers providing evidence that a genetic variation associated with Alzheimer’s may also be affecting the severity of pathology in Parkinson’s. The genetic variation associated with Alzheimer’s occurs in a gene (a functional region of DNA) called ApoE, and the Parkinson’s pathology involves the clustering of a protein called alpha synuclein. Specifically, both researchers reported that a genetic variation called ApoE4 is associated with higher levels of alpha synuclein clustering. And ApoE4 is also associated with worse cognitive issues in people carrying it. In today’s post, we will discuss what ApoE is, what is known about ApoE4, what these new studies found, and what it could mean for the future treatment of Parkinson’s and associated conditions.
|
 A mutant. Source: Screenrant
A mutant. Source: Screenrant
When I say the word ‘mutant’, what do you think of?
Perhaps your imagination drifts towards comic book superheroes or characters in movies who have acquired amazing new super powers resulting from their bodies being zapped with toxic gamma-rays or such like.
Alternatively, maybe you think of certain negative connotation associated with the word ‘mutant’. You might associate the word with terms like ‘weirdo’ or ‘oddity’, and think of the ‘freak show’ performers who used to be put on display at the travelling carnivals.
 Circus freak show (photo bombing giraffe). Source: Bretlittlehales
Circus freak show (photo bombing giraffe). Source: Bretlittlehales
In biology, however, the word ‘mutant’ means something utterly different.
What does ‘mutant’ mean in biology?
The word ‘mutant’ comes from the Latin mutāre which means “to change”.
In biology, we refer to a mutant when talking about small changes in DNA (which can affect the subsequent protein, which can also be referred to as a ‘mutant protein’). Many researchers prefer the words genetic variations, as the changes (or mutation) observed in DNA can vary greatly in size and nature.
 Source: News-medical
Source: News-medical
It is important to understand, however, that there is nothing special about these genetic variations.
In fact, the natural random occurrence of genetic mutations is integral to the process of evolution. And every single one of us carries genetic variations – we are each a genetic experiment. A few years back, researchers found that when parents pass their genes on to their offspring, an average of 60 new completely random mutations are introduced into their child’s DNA:
 Title: Variation in genome-wide mutation rates within and between human families.
Title: Variation in genome-wide mutation rates within and between human families.
Authors: Conrad DF, Keebler JE, DePristo MA, Lindsay SJ, Zhang Y, Casals F, Idaghdour Y, Hartl CL, Torroja C, Garimella KV, Zilversmit M, Cartwright R, Rouleau GA, Daly M, Stone EA, Hurles ME, Awadalla P; 1000 Genomes Project.
Journal: Nat Genet. 2011 Jun 12;43(7):712-4.
PMID: 21666693 (This report is OPEN ACCESS if you are interesting in reading it)
But what is actually meant by these small genetic variations? What exactly is a mutation in DNA?
To explain this, we need to understand a little something about basic genetics.
In almost every cell of your body you have DNA (or Deoxyribonucleic acid). It is a molecule that is composed of two chains which coil around each other, forming the famous double helix shape.
 Source: Pngtree
Source: Pngtree
The information stored in DNA provides the template – or the instructions – for making (and maintaining) a particular organism. All of the necessary details are encoded in that amazing molecule.
How is information stored in DNA?
In the image above, you can see strands reaching between the two chains of the DNA molecule, and it is these numerous strands that contain the information.
These strands are made up of a pair of ‘nucleotides’. These are organic molecules which contain one of four bases – the familiar A, C, T & Gs that make up our DNA code. These nucleotides pairings (called ‘base pairs’) join together in long strings of DNA.
 The basics of genetics. Source: CompoundChem
The basics of genetics. Source: CompoundChem
It takes a lot of instructions to make a fully functional human being, and as a result human DNA contains a lot of these base pairs – 3 billion of them. And having so many base pairs results in a lot of DNA in each cell – 2 meters of it. In order to get all that DNA crammed into the nucleus of a tiny little cell, DNA is wound up tightly into thread-like structures called chromosomes.
 DNA in a chromosome. Source: Byjus
DNA in a chromosome. Source: Byjus
Humans have 23 pairs of chromosomes. And the genetic mutations we are talking about today can occur in any of these chromosomes.
This video does a better job of explaining DNA than I do:
Now, if DNA provides the template for making a human being, it is the small variations in our individual DNA that help to make each of us unique. Every single one of us has small genetic variations.
And these variations come in different flavours: some can simply be a single mismatched base pair (also called a point-mutation or single nucleotide polymorphism (SNP)), while others are more complicated such as repeating copies of multiple base pairs.

Lots of different types of genetic variations. Source: Nature
An example of how these variation can make us who we are is red hair. The fact that an individual has red hair results (in the majority of cases) from a variation in a region of DNA (called MC1R) on chromosome 16.
If that variation is not there: no red hair.
Most of the variants that we have that define who we are, we have had since conception. These are called ‘germ line’ mutations, while those that we pick up during life and that are usually specific to a particular tissue or organ in the body (such as the liver or blood), are called ‘somatic’ mutations.

Somatic vs Germline mutations. Source: AutismScienceFoundation
These variations can occur in regions of DNA that have no apparent use (about 90% of your DNA), but they can also appear in more functional regions (which are called genes). Genes are regions of DNA that provide the instructions for producing proteins, enzymes and pieces of regulatory RNA. These regions provide the instructions (or RNA), which can in some cases be used to make protein. Where genetic mutations fall within these regions, the resulting RNA and protein can carry a variation that will impact their function – sometimes increasing activity, sometimes preventing activity.
An important aspect of these variations in genes is that you have two copies of each gene (called alleles). One copy from your father, and one copy from your mother. Call it mother nature’s insurance policy – if one copy of a gene is faulty, you have a back up. But sometimes one copy is not enough, and this can lead to problems.
In conditions that are influenced by genetics, there are two types of inherited mutations:
- A variant has to be provided by both the parents for a condition to develop – this is called an ‘autosomal recessive‘ disease. In this case, both copies (or alleles) will be mutated, resulting in the condition having a higher chance of developing. An example of this is cystic fibrosis (a condition in which sticky mucus builds up in the lungs and digestive system).
- Only one copy of the variant needs to be provided by one of the parents for a condition to develop – this is called an ‘autosomal dominant’ disease. In this case just one allele is required for a condition to have a higher chance of developing, and a example of this is the neurodegenerative condition of Huntington’s.

Autosomal dominant vs recessive. Source: Wikipedia
It is important to understand that most of these tiny genetic variations result in no impact on an organism. The region of DNA is not important, or biology is able to adapt and find a way around the problem.
Other variants can infer traits that may be considered beneficial, for example, some people have a genetic mutation in the CCR5 gene. HIV uses the CCR5 protein to enter into human cells. Thus, if a person has a mutation in their CCR5 gene, they are extremely unlikely to become infected by the HIV virus.
 Source: Quora
Source: Quora
There are, however, some genetic variation that are of a more serious nature – leaving us potentially vulnerable to developing a condition (such as BRCA1 mutations in the case of breast cancer).
|
RECAP #1: Genetic variations – tiny differences in our DNA – between individuals is an important part of what makes each of us unique. Some of these variations can also make us more vulnerable to certain conditions.
|
In addition to these ‘vulnerability’ variations, we are now learning about others that are associated with particular aspects of a condition, such as the severity of a condition or speed of progression, once the aliment has already developed.
Over the last two decades, researchers have identified not only genetic risk factors for developing a particular conditions, but also genetic variations that can influence certain characteristics of the condition itself.
And some of these varations appear to be shared across conditions as well.
Can you give me an example?
Yes, let’s use ApoE4 as an example.
What is ApoE4?
Apolipoprotein E4 (also known as apoE4) is the most prevalent genetic risk factor of Alzheimer’s. It is present in more than half of Alzheimer’s patients.
What is ApoE4?
Apolipoprotein E is a protein that is involved in the transportation of lipids and injury repair in the brain.
Outside of the brain, ApoE is primarily produced by the liver and macrophage cells in the blood. It functions by mediating cholesterol metabolism.
The apolipoprotein E gene sits on chromosome 17 of our DNA.
Hang on a second. What happened to the “4” in ApoE4? Why did you shift from ApoE4 to ApoE?
Because ApoE is the gene, but it is “polymorphic“.
And what does that mean?!?
Polymorphic means that there are two or more clearly different morphs (or versions/forms) of the gene due to variations.
So everyone doesn’t have the same ApoE gene?
No, there are three common versions/forms (or morphs) of the gene:
- ApoE2,
- ApoE3
- ApoE4
These different forms of the ApoE gene result in tiny differences in the apoE protein (only one or two amino acids at positions 112 and 158). But these differences alter the activity of the protein and how they interact with the world.
Now you may remember that everyone inherits two copies of a gene, one from each parent. Having two copies of each gene is mother nature’s insurance policy that an organism will have an increased chance of surviving. But sometimes those two copies of the same gene can vary slightly.
Therefore it is possible for you to have two different versions of the ApoE gene. You could have any one of the following six combinations (or “genotypes”):
- E2/E2 (which occurs in 1% of the UK population)
- E2/E3 (11%)
- E2/E4 (2%)
- E3/E3 (61%)
- E3/E4 (23%)
- E4/E4 (2%)
Individuals carrying any combination of the ApoE4 gene are at increased risk of developing Alzheimer’s, compared with people carrying the more common ApoE3 gene or the rarer ApoE2 gene (the ApoE2 gene actually decreases risk).
 Source: PMC
Source: PMC
And before we go on it is important to understand that these genetic variations are simply an “association” with increased risk. They do not necessarily mean that someone with two copies of ApoE4 will definitely develop Alzheimer’s. That individual may not.
In addition to these ApoE4 variations there are probably dozens of counter balancing genetic variants that may reduce the risk of Alzheimer’s. Our knowledge of the genetics of these conditions still requires a lot of work.
|
RECAP #2: Apolipoprotein E (ApoE) is a protein that is involved in the transporting material around in cells and injury repair in the brain. There are different versions of the protein. People with the ApoE4 version have an increased risk of developing Alzheimer’s.
|
Interesting, but what does any of this have to do with Parkinson’s?
Well, very recently two research groups published data suggesting that this ApoE gene may not just play a role in Alzheimer’s, but it may also be influential in Parkinson’s.
The two research groups published back-to-back reports (meaning that they followed each other in the same journal).
Here is the first report:
 Title: APOE genotype regulates pathology and disease progression in synucleinopathy.
Title: APOE genotype regulates pathology and disease progression in synucleinopathy.
Authors: Davis AA, Inman CE, Wargel ZM, Dube U, Freeberg BM, Galluppi A, Haines JN, Dhavale DD, Miller R, Choudhury FA, Sullivan PM, Cruchaga C, Perlmutter JS, Ulrich JD, Benitez BA, Kotzbauer PT, Holtzman DM.
Journal: Sci Transl Med. 2020 Feb 5;12(529). pii: eaay3069.
PMID: 32024799
In this study, the investigators the researchers genetically engineered mice with the A53T mutation in the alpha synuclein gene.
What does that mean?
On this website we are forever talking about a protein called alpha synuclein.
It sounds like a distant galaxy, but it is one of the most common proteins in our brains. It makes up about 1% of all the protein in a neuron. When alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’, in that is does not really have a defined structure. When it is first produced, alpha synuclein will look something like this:

Alpha synuclein. Source: Wikipedia
In this form, alpha synuclein is considered a monomer – which is a single molecule that can bind to other molecules. When it does bind to other alpha synuclein proteins, they form an oligomer (a collection of a certain number of monomers in a specific structure). It is believed that alpha synuclein has certain functions as a monomer, but may also have specific tasks as a oligomer.
In Parkinson’s, alpha synuclein also binds (or aggregates) to form what are called ‘fibrils’.

Microscopic images of alpha synuclein (AS) monomers, oligomers and fibrils. Source: Brain
And it is believed that the oligomer and fibril forms of alpha synuclein protein that aggregate together, and then go on to form what we call Lewy bodies.
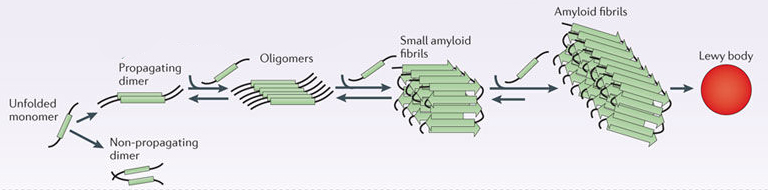 Parkinson’s associated alpha synuclein. Source: Nature
Parkinson’s associated alpha synuclein. Source: Nature
What are Lewy bodies?
Lewy bodies are dense circular clusters of aggregated alpha synuclein protein (and other proteins) that are found in specific regions of the brain in people with Parkinson’s (Click here for more on Lewy bodies).
Lewy bodies are one of the cardinal features of the Parkinsonian brain – they are used to help make postmortem diagnoses.
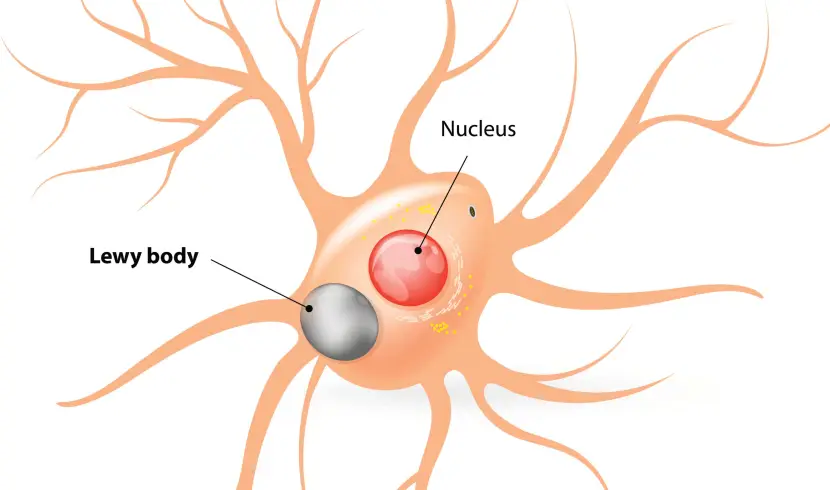 A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
A cartoon of a neuron, with the Lewy body indicated within the cell body. Source: Alzheimer’s news
Ok, but what is the “A53T mutation” part all about?
The section of DNA that gives rise to alpha syncuclein protein is called SNCA.
And there are several genetic variations inside of SNCA that are associated with an increased risk of developing Parkinson’s. A53T is the name of one of those genetic variations.
As you can see in the image below, A53T lies in the red (Amphipathic) region of SNCA along with several other genetic variants, such as A30P and E46K:
Mice have been genetically engineered to carry the human SNCA gene with the A53T genetic mutation (Click here to read the original report). These mice initially exhibit hyperactivity and then start to display signs of alpha synuclein protein accumulation and aggregation at about four to six months of age. They also pass away earlier than normal mice (12-14 months of age, compared to 20+ months for normal mice).
I see. So what did the researchers do with the mice?
In addition to the A53T mutation in the alpha synuclein gene, the investigators also engineered the mice so that they carried either no ApoE gene or one of the 3 human APOE variations (ApoE2, ApoE3, or ApoE4).
They then bred these “mutant alpha synuclein+ApoE variant” mice and watched to see what happened. At 12 months of age, the researchers found that the mice carrying the ApoE4 version of the ApoE gene had higher levels of alpha synuclein than the rest of the mice.
In fact, the ApoE2 mice had undetectable levels of alpha synuclein. In addition, the ApoE2 mice survived longer, while the ApoE4 mice died ealier. The ApoE4 mice also had more motor problems than the ApoE2 mice.
On top of the mouse research, the investigators also looked at two independent clinical cohorts of people with Parkinson’s and they found that individuals with two copies of the ApoE4 gene exhibited faster rates of cognitive decline compared with people with Parkinson’s who do not have a copy of ApoE4.
And the second study found very similar results:
 Title: APOE4 exacerbates α-synuclein pathology and related toxicity independent of amyloid.
Title: APOE4 exacerbates α-synuclein pathology and related toxicity independent of amyloid.
Authors: Zhao N, Attrebi ON, Ren Y, Qiao W, Sonustun B, Martens YA, Meneses AD, Li F, Shue F, Zheng J, Van Ingelgom AJ, Davis MD, Kurti A, Knight JA, Linares C, Chen Y, Delenclos M, Liu CC, Fryer JD, Asmann YW, McLean PJ, Dickson DW, Ross OA, Bu G.
Journal: Sci Transl Med. 2020 Feb 5;12(529). pii: eaay1809.
PMID: 32024798
In this study, the researchers used viruses to deliver alpha synuclein to mice.
How did they do that?
Viruses are simple things.
They are a basically a shell with a bit of DNA inside it. The shell attaches to a cell, injects its DNA and the cell then produces more copies of the virus. Very simple, right?
Now, if you take the viral DNA out of the shell of the virus, what you have left is a very effective biological delivery system – one which can be used to delivery any kind of DNA you want.
Researchers have embraced this idea and used viruses as research tools. And in the current study, the investigators used viruses to deliver alpha synuclein DNA (called SNCA) into the brains of mice which carried either the ApoE2, ApoE3, or ApoE4 versions of ApoE.
They found that mice carrying ApoE4 had worse alpha synuclein pathology, and more behavioral problems than mice with ApoE2 or ApoE3.
And these researchers also looked at humans, but they focused their analysis on postmortem brain samples. They assessed levels of alpha synuclein pathology in section of brain from people who passed away with Lewy Body Dementia (Click here to read a previous SoPD post about this topic). Specifically, the researchers compared samples from people carrying ApoE4 vs non-carriers, and they reported more severe alpha synuclein pathology in the ApoE4 carriers.
Both groups of researchers concluded their studies by suggesting that the version of ApoE each of us carries can regulate alpha synuclein pathology.
What does this mean if I am an ApoE4 variant carrier?
As we discussed above, these results are based on an association, and not all individuals with ApoE4 variants go on to develop Alzheimer’s (and the case is most likely the same with alpha synuclein pathology). So there is no reason to immediately start panicking.
All of this research needs to be further explored and expanded on.
|
RECAP #3: Two groups of researchers found that mouse models of Parkinson’s were more severe in mice carrying the ApoE4 genetic variation, than ApoE2 or ApoE3 versions of the gene. The investigators also looked in humans and found that people with Parkinson’s or associated conditions who also carried the ApoE4 variation, have a great burden of protein aggregation in their brains and displayed faster rates of cognitive decline.
|
Interesting. So what does it all mean?
No, we’re not finished yet.
Given the prominent role of ApoE4 in Alzheimer’s, there has been a great deal of research exploring it as a therapeutic target. And many different methods have been proposed for reducing the effect of this version of ApoE (for those interested in this particular topic, click here for a very good recent review of the different approaches to targeting ApoE4).
IF ApoE4 is found to be having an influential role in alpha synuclein-associated conditions, then this means we could start exploring some of these ApoE4 targetted experimental therapies in certain sub-groups of neurodenerative conditions other than Alzheimer’s.
One approach to reducing the influence of ApoE4 is the use of immunotherapy.
What is immunotherapy?
Immunotherapy involves boosting the body’s immune system to target specific toxic agents in the body. In the case of Parkinson’s, this approach is primarily being focused on different forms of alpha synuclein.

Antibodies. Source: Astrazeneca
The immunotherapy approach uses antibodies, which are Y-shaped proteins that act like alert flags for the immune system. Once enough antibodies bind to a particular object, the immune system will dispose of it. Antibodies target very specific structures, while ignoring everything else.
In Parkinson’s, the immunotherapy approaches are primarily involving antibodies that target the alpha synuclein protein. By tagging the alpha synuclein as it is being passed from one cell to another, and allowing the immune system to remove it, researchers hope to slow down the progression of Parkinson’s (Click here to read a previous SoPD post on this topic).
How could immunotherapy be used to reduce the influence of ApoE4?
This research report was published in 2018 and it caused a lot of interest:
 Title: Targeting of nonlipidated, aggregated apoE with antibodies inhibits amyloid accumulation.
Title: Targeting of nonlipidated, aggregated apoE with antibodies inhibits amyloid accumulation.
Authors: Liao F, Li A, Xiong M, Bien-Ly N, Jiang H, Zhang Y, Finn MB, Hoyle R, Keyser J, Lefton KB, Robinson GO, Serrano JR, Silverman AP, Guo JL, Getz J, Henne K, Leyns CE, Gallardo G, Ulrich JD, Sullivan PM, Lerner EP, Hudry E, Sweeney ZK, Dennis MS, Hyman BT, Watts RJ, Holtzman DM.
Journal: J Clin Invest. 2018 May 1;128(5):2144-2155.
PMID: 29600961 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers genetically engineered mice to produce human ApoE4 and high levels of the Alzheimer’s associated protein beta amyloid. They then treated some of these mice with an antibody for human ApoE4 and they found that the antibodies decreased the accumulation of beta amyloid aggregates in the brain.
Of particular interest is that this research was supported by a biotech company called Denali:
Regular readers will be aware that Denali are leading the pack with regards to developing LRRK2 inhibitors for Parkinson’s (Click here to read a previous SoPD post about this). Denali do not mention ApoE4 immunotherapy on their pipeline page (Click here to see that page), so it may still be considered very early in development, but something to keep an eye out for.
So what does it all mean?
Some of the tiny variations in our DNA that make us each unique can play an important how we respond to certain diseases. These mutations can make us vulnerable to some conditions, while strengthening us againsts others. Recently researchers have reported that a variation associated with Alzheimer’s – ApoE4 – may also be influential in Parkinson’s and associated conditions.
More research is needed in this area, but if the findings are replicated and expanded on, there is the opportunity to piggy-back on some of the Alzheimer’s-related research that has been conducted in this area. And we may also see new therapies that have been designed to target the ApoE4 protein being used in the treatment of people with Parkinson’s who also carry this genetic variation.
One step closer to a more personalised approach.

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from Youtube




For anyone wanting to dive deeper, but unable to access the two key research papers, AlzForum has a pretty solid article covering both papers:
https://www.alzforum.org/news/research-news/toxic-synuclein-egged-apoe4-thwarted-apoe2
LikeLike