|
# # # # This week we received news of a case study involving a cell transplantation procedure that was performed during 2017/2018 on an American gentleman with Parkinson’s. The operation (conducted in in Boston, USA) involved isolating skins cells from the individual who under went the surgery, then converting those cells into stem cells which were further encouraged to become dopamine neurons before being transplanted into his brain. Although this is a single subject study (no control group), the result suggests that 2 years on the procedure is safe and well tolerated. In today’s post, we will discuss the background of this research, review the published results, and explore other aspects of this story. # # # # |
 Source: Medium
Source: Medium
On the 12th May, a story was posted on the online news site, STATnews.
I like STATnews (not an endorsement, just me sharing). They have lots of interesting stories covering a wide range of health and biotech topics.
But the story on the 12th May was different.
It focused on clinical study that involved just one participant – a gentleman from Southern California who was afflicated by Parkinson’s. He underwent a procedure called cell transplantation (Click here to read the STATnews story).
What is cell transplantation?
(A lot of what we are about to discuss was covered in the recent post on Aspen Neuroscience (Click here to read that post). If you read that post, skip down to Recap #2).
By the time a person is presenting the motor features characteristic of Parkinson’s, and being referred to a neurologist for diagnosis, they have already lost approximately 50% of the dopamine producing neurons in an area of the brain called the substantia nigra, which resides in a region called the midbrain.
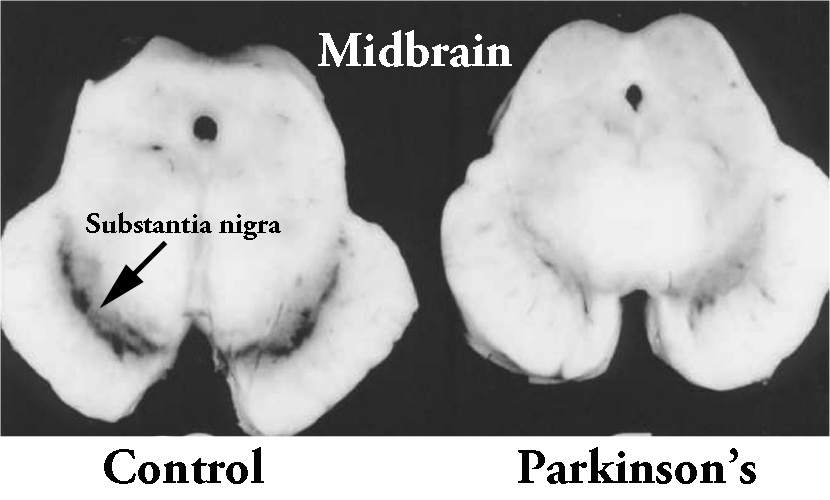 The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
These cells are critical for normal motor function – without them, movement becomes very inhibited.
And until we have developed methods that can identify Parkinson’s long before these cells are lost and the motor features appear, some form of cell replacement therapy is required to introduce new dopamine cells to take up the lost function.
Cell transplantation represents the most straight forward (but still experimental) method of cell replacement therapy.
How does cell transplantation work?
There are basically two approaches to cell transplantation: the ‘old fashioned’ and the ‘new stem cell-based’ methods.
This ‘old fashioned’ approach to cell transplantation has involved dissecting out the region of the developing dopamine neurons from a donor embryo, breaking up the tissue into small pieces that could be passed through a tiny syringe, and then injecting those cells into the brain of a person with Parkinson’s.

The old cell transplantation process for Parkinson’s. Source: The Lancet
Critically, the people receiving this sort of transplant have required ‘immunosuppression treatment’ for a long period of time after the surgery. This additional treatment involves taking drugs that suppress the immune system’s ability to defend the body from foreign agents. This step is necessary, however, in order to stop the body’s immune system from attacking the transplanted cells (which would not be considered ‘self’ by the immune system), allowing those cells to have time to mature, integrate into the brain and produce dopamine.
The transplanted cells are injected into an area of the brain called the putamen.
What is the putamen?
The bulk of the dopamine neurons in the brain reside in an area called the substantia nigra, near the base of the brain, but they project their branches (or axons) to the several other areas, including the putamen, and this is where they release most of their actual dopamine (the chemical which helps us to move properly).
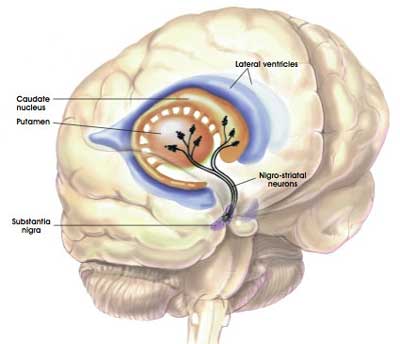 The projections of the substantia nigra dopamine neurons & location of the putamen. Source: MyBrainNotes
The projections of the substantia nigra dopamine neurons & location of the putamen. Source: MyBrainNotes
In people with Parkinson’s, the amount of dopamine being released in the putamen decreases over time. The image below demonstrates the loss of dopamine (the dark staining) over time as a result of Parkinson’s (PLEASE NOTE that the time scale presented here varies from person to person):
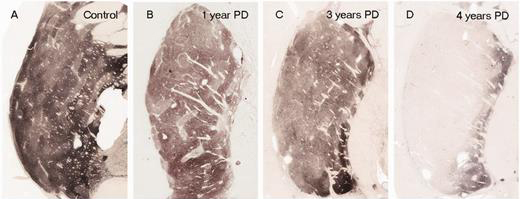
The loss of dopamine in the putamen as Parkinson’s progresses. Source: Brain
In cell transplant procedures for Parkinson’s, multiple injections of cells are usually made in the putamen, allowing for deposits in different areas of the structure. These multiple sites allow for the transplanted cells to produce dopamine across the entire extent of the putamen. And ideally, the cells should remain localised to the putamen, so that they are not producing dopamine in areas of the brain where it is not desired (possibly leading to side effects).

Targeting transplants into the putamen. Source: Intechopen
Postmortem analysis of the brains of individuals who have previously received transplants of dopamine neurons (and then subsequently died from natural causes) has revealed that the transplanted cells can survive the surgical procedure and integrate into the host brain. In the image below, you can see rich brown areas of the putamen in panel A. These brown areas are the dopamine producing cells. A magnified image of individual dopamine producing neurons (their circular bodies and their branches are stained in brown) can be seen in panel B:

Transplanted dopamine neurons. Source: Sciencedirect
The transplanted cells take several years to develop into mature neurons after the transplantation surgery. This means that the actually benefits of the transplantation technique will not be apparent for some time (2-3 years on average). Once mature, however, it has also been demonstrated (using brain imaging techniques) that these transplanted cells can produce dopamine.
As you can see in the images below, there is less dopamine being processed (indicated in red) in the putamen of the Parkinsonian brain on the left than the brain on the right (several years after bilateral – both sides of the brain – transplants):

Brain imaging of dopamine processing before and after a successful transplantation. Source: NIH
In some cases the individual being transplanted has been able to reduce the amount of Levodopa treatment that they take over time. As the transplanted cells start to produce enough dopamine in the right area of the brain, it allows the individual to function better and require less medication.
The ‘old fashioned’ approach to cell transplantation involved dissecting out the region of the developing dopamine neurons from a donor embryo, and for obvious ethical reasons the research community has been shifting away from this method and focusing their attention on ‘new stem cell-based’ methods.
By expanding stem cells in culture, the scientists have an endless supply of cells to work with.

Stem cells in a petridish. Source: Wikipedia
And sophisticated protocols now allow them to encourage the stem cells to mature into bona fide dopamine neurons – the exact type of cell that are required for transplantation.
There are numerous research groups around the world who are now attempting to take this stem cell-based form of cell transplantation to the clinic for Parkinson’s. Many of those researchers are part of a consortium called G-FORCE.
In 2019, The Cure Parkinson’s Trust supported a meeting of the G-FORCE consortium that was held in Cambridge UK. This video gives you a summary of the meeting and the G-FORCE group:
For those of you who are interested, there are videos of presentations made by G-FORCE researchers who attended the meeting on the Cure Parkinson’s Trust website (Click here to view them).
And here is one example of one of the presentations from Prof Roger Barker (University of Cambridge):
|
# RECAP #1: Parkinson’s is a neurodegenerative condition, which means that cells are being lost in the brain. Cell transplantation represents an experimental approach to replacing some of those lost cells. It typically involves injections of dopamine producing cells into a region of the brain called the putamen, which is where the chemical dopamine has it’s action. # |
Ok, so the STATnews story described a study involving a single guy getting a cell transplantation procedure. What was so special about that study?
It was the type of cells that were transplanted that made this study special. The cells represent a new type of stem cell called induced pluripotent stem cells (or IPS cells), and this was the first time they had been transplanted into a human brain.
What are induced pluripotent stem cells?
Pluripotent stem cells are cells that can be grown (in cell culture) to become any type of cell in the body – heart, brain, bone, blood, etc. ‘Pluripotent’ meaning capable of any fate. These cells start off in a naive state, but using specific protocols (or recipes) of certain proteins, these cells can be encouraged to differentiate into any kind of cell we may require.
 The menu. Source: Wikipedia
The menu. Source: Wikipedia
But it is the “induced” part of their name that makes these pluripotent stem cells special.
This is Prof Shinya Yamanaka.

Prof Shinya Yamanaka. Source: Glastone Institute
He’s a rockstar in the biomedical research community.
Prof Yamanaka is the director of Center for induced Pluripotent Stem Cell Research and Application (CiRA); and a professor at the Institute for Frontier Medical Sciences at Kyoto University.
But more importantly, in 2006 he published a research report that would quite literally ‘change everything’.
This is the report:

Title: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors.
Authors: Takahashi K, Yamanaka S.
Journal: Cell. 2006 Aug 25;126(4):663-76.
PMID: 16904174 (This article is OPEN ACCESS if you would like to read it)
In this study, Prof Yamanaka‘s team demonstrated a method by which someone could take a simple skin cell (called a fibroblast), grow it in cell culture for a while, and then re-program it so that it would transform back into a stem cell – a cell that is capable of becoming any kind of cell in the body.
So rather than just being a skin cell that could divide into other skins cells, the transformed (or “induced”) ‘stem cell’ could now become any kind of cell. It was pluripotent.
And because they had persuaded the skin cells to change back to this immature state, the researchers decided to call the transformed cells induced pluripotent stem (or IPS) cells.
Prof Yamanaka‘s team started their investigation with the basic hypothesis that proteins which are important to the maintenance of embryonic stem cells (the cells that give rise to all of the cells in your body) might also be able to cause an embryonic state in mature adult cells. They selected twenty-four proteins that had been previously identified as important in embryonic stem cells to test this idea. They used re-engineered viruses to deliver these proteins to mouse skin cells. The viruses were emptied of all their disease causing properties, and could thus function as very efficient biological delivery systems.
The skin cells were genetically engineered in such a fashion so that only cells in which reactivation of the embryonic stem cells-associated protein, Fbx15, would survive the testing process. If Fbx15 was not turned on in the cells, over time they would die. When the researchers infected the cells with all twenty-four embryonic stem cells genes, remarkably some of the cells actually survived and began to divide like stem cells.
In order to identify which proteins were necessary for the reprogramming, the researchers began removing one protein at a time from the pool of twenty-four. Through this process, they were able to narrow down the most effective proteins to just four: Oct4, Sox2, cMyc, and Klf4, which became known as the “Yamanaka factors”.
Interesting. But why is this important?
Understand that this was more than an amazing feat of molecular biology. It suddenly made the hypothetical idea of ‘personalised medicine’ very possible – take skin cells from anyone with a particular medical condition, turn them into whatever cell type you like, and then either test drugs on those cells or transplant them back into their body (replacing the cells that have been lost due to the medical condition).
Personalised medicine with IPS cells. Source: Bodyhacks
IPS cells are now being used all over the world, for all kinds of biomedical research. And many research groups (including those involved in the G-FORCE consortium) are working to bring IPS cell-based therapies to the clinic in the hope of providing the long sought-after dream of personalised medicine.

Making IPS cells. Source: learn.genetics
For example, we can now take skins cells from a person with Parkinson’s and turn those cells into dopamine neurons which can then be tested with various drugs to see which treatment is most effective for that particular person (personalised medicine in it’s purest form).

Some of the potential options available to through IPS cells. Source: Nature
Some of those dopamine neurons could also potentially be transplanted back into the person they were originally collected from – into the brain to replace the lost dopamine neurons. This process would hopefully reduce the need for medication that suppresses the immune system, because the body would recognise the cells being transplanted as ‘self’ (or derived from the person being transplanted).
And this is exactly what the study described in the STATnews story did.
|
# # RECAP #2: Induced pluripotent stem cells (or IPS cells) are dividing cells that are derived from mature cells (such as skin cells) via a feat of molecular manipulation. After being re-programmed to an immature state, these cells are capable of becoming any type of cell in the body. They can be used in Parkinson’s research for personalised in vitro drug testing or potentially for cell transplantation. The attractive aspect of IPS cells for cell transplantation is that the cells can be collected from the individual to be transplanted, which will hopefully reduce the need for immunosuppression as the body will recognise the transplanted cells as “self” rather than as foreign. # # |
So what did they find in this new study?
Two days after the STATnews story highlighted this study, this short report appeared in the New England Journal of Medicine:
 Title: Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease.
Title: Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease.
Authors: Schweitzer JS, Song B, Herrington TM, Park TY, Lee N, Ko S, Jeon J, Cha Y, Kim K, Li Q, Henchcliffe C, Kaplitt M, Neff C, Rapalino O, Seo H, Lee IH, Kim J, Kim T, Petsko GA, Ritz J, Cohen BM, Kong SW, Leblanc P, Carter BS, Kim KS.
Journal: N Engl J Med. 2020 May 14;382(20):1926-1932.
PMID: 32402162
This report described the study that was discussed in the STATnews story. In their study, the researchers collected skin cells (via a skin biopsy) from a 69-year-old man who was diagnosed with Parkinson’s 10 years ago. They converted these cells into IPS cells using a protocol that they recently described and tested.
A report of that protocol was published earlier this year:
 Title: Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models.
Title: Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models.
Authors: Song B, Cha Y, Ko S, Jeon J, Lee N, Seo H, Park KJ, Lee IH, Lopes C, Feitosa M, Luna MJ, Jung JH, Kim J, Hwang D, Cohen BM, Teicher MH, Leblanc P, Carter BS, Kordower JH, Bolshakov VY, Kong SW, Schweitzer JS, Kim KS. J
Journal: Clin Invest. 2020 Feb 3;130(2):904-920.
PMID: 31714896 (This report is OPEN ACCESS if you would like to read it)
In this previous report, the researchers described the developed and testing of a method to efficiently generate clinical-grade IPS cells (exploring both genomic integrity and the unbiased pluripotent potential of the cells). They also established a “spotting”-based method for cell culture differentiation of healthy dopamine neurons in a scalable manner. In addition, they also described a chemical method that safely allowed for the elimination of undifferentiated cells from the final population that is going to be transplanted (transplanting undifferentiated cells could give rise to the incorrect type of cells or – worse – uncontrolled stem cell expansion in the brain).
Applying these methods to the cells collected from the 69-year-old man in the present study, the researchers were able to generate IPS cells which they then characterised in cell culture and after transplanting into the brains of immunodeficient mice.
Satisfied with their characterisation results, the researchers next expanded these patient-derived IPS cells, differentiated them into dopamine neurons, and then transplanted into the brain of the 69 year old patient. The gentleman underwent two MRI-guided surgical operations. First, the researchers implanted cells in the putamen on the left side of the brain, and then 6 months later, he was transplanted on the right side of the brain. Each operation involved cells being deposited along three trajectories in the putamen.

Targeting transplants into the putamen. Source: Intechopen
A total of 4 million cells were transplanted on each side of the brain (divided equally among the three tracks). After each surgery, the patient was kept in hospital and monitored overnight, before being discharged the next day. At no point in the study were any immunosuppressants used.
The investigators then monitored and assessed the patient for two years (after the first operation). Importantly, there were no adverse events associated with the procedure, so the operation was apparently safe and well tolerated. The researchers reported that there were subtle changes in brain imaging data (PET 18F-DOPA uptake) compared to baseline images. In addition, with regards to clinical measures of Parkinson’s, the researchers reported that after surgery symptoms “stabilized or improved at 18 to 24 months”.
EDITOR’S NOTE: It is important to remember that this was a single patient, open-label case study, so a placebo response may have been in effect with regards to the clinical outcomes. Thus, we should not read too much into the clinical results.
At 24 months post first surgery, the patient had decreased his levodopa equivalent medication by 6% (compared to before the transplantations). He also reported that his OFF periods were less than 1 hour per day and no dyskinesias had been experienced (or observed during clinical assessments – which was similar to his pre-surgery state).
The investigators concluded that “further studies are warranted to address how this approach will perform in a variety of patients with diverse genetic backgrounds and disease phenotypes over a period longer than 24 months“.
|
# # # RECAP #3: A case study of a single individual with Parkinson’s being transplanted with his own IPS cell-derived dopamine neurons has been published. Two years post surgery, no adverse events have been reported and there are indications that his clinical features have stabilised. Given that this is a single participant, open-label study it is difficult to take much away from the study beyond the finding that the procedure was safe and tolerated (out to two years). # # # |
So summin’ up? What does it all mean?
No, we’re not finished just yet.
There is another aspect to this story that needs to be discussed.
Que?
On the same day that the paper was published by the New England Journal of Medicine, a new report was posted on the STATnews website, written by the same author of the previous post. But this follow up story reported that ethics questions were now swirling “around historic Parkinson’s experiment”.
The questions – according to the STATnews follow up report – dealt with:
- Scientific integrity – specifically, concerns have been raised regarding the fact that the individual who underwent the surgery, not only paid for the research but also recruited the neurosurgeon to do the transplant.
- Science bending to the rich – questions have also been asked regarding if we are entering a new phase where “academic medical centers might move their research to where the money is, not where the greatest scientific promise and medical need are”?
- Secrecy – many in the research community were completely unaware of it until the study was published this week (32 months after the first surgery). The investigators go to great lengths in their report to state that everything was done according to ethical board & regulator guidelines, but some scientients are wondering why the researchers chose to wait 32 months (after the first surgery was conducted) before sharing news of the operation with the research community.
Now, I am uncomfortable with this situation from several angles (such as why STATnews hyped the story one day and questioned it the next?), but I am sharing a summary of the research report here as I know there is great interest in the field of cell transplantation within the Parkinson’s community.
While I wish that the researchers had included several additional participants in their study – which would have balanced out some of the concerns raised above – and that they had shared their project with groups like the G-FORCE consortium, I hope that the gentleman who received the treatment responds well and has a better quality of life.
There will be some who question the ethics of this situation, but it is important to remember that the knowledge generated from the research that this individual funded will hopefully benefit everyone. The question could be asked “how is this story different from cases where wealthy individuals start biotech companies to find treatments for their child’s rare disease?”
Here on the SoPD, we have previously discussed the idea of a “plutocratic proposal” – or wealthy individuals paying for clinical trials (Click here to read that post), but ideally those trials should involve more than one participant (reducing the ethical risk for the researchers and institutions also involved).
Do we know what they researchers are planning to do next?
No.
At present I have not heard if they plan any further clinical trials. One would assume that this work would represent a foundation that could be built upon.
As discussed above, however, other research groups are conducting (or preparing to start) clinical trials for IPS cell-based approaches. There is an IPS cell trial for Parkinson’s being conducted in Kyoto (Japan) – being led by Prof Jun Takahashi (Click here to read a previous SoPD post on this topic).
 Prof Jun Takahashi (speaking) and colleagues. Source: Japantimes
Prof Jun Takahashi (speaking) and colleagues. Source: Japantimes
In addition, there are multiple biotech companies now preparing clinical trials for IPS cell-based cell transplantation in Parkinson’s. The most advanced of these are the biotech firms Aspen Neuroscience and BlueRock Therapeutics (Click here and here to read previous SoPD posts about these efforts).
 Hopefully later this year we should hear news about the Kyoto study (2 years on from the first surgeries) and – fingers crossed – the initiation of additional clinical trials by BlueRock or Aspen.
Hopefully later this year we should hear news about the Kyoto study (2 years on from the first surgeries) and – fingers crossed – the initiation of additional clinical trials by BlueRock or Aspen.
EDITORS NOTE: The Kyoto researchers and scientists involved with BlueRock and Aspen are all active members of the G-FORCE consortium – sharing their research and progress.
So what does it all mean?
At the end of the second STATnews report, renowned stem cell researchers Prof Jeanne Loring states that “There is a lot riding on this” – referring to cell therapy for Parkinson’s. And she is correct in several ways. Previous missteps have held the field of cell transplantation back for decades and similar situations now could have massive implications for research groups trying to move forward in this space.
And the reporting of these research efforts could also affect the overall picture as well. Thus, questions should be asked when a news/media organisation highlights a scientific report one day, before questioning it the next. A badly timed coincidence? Or an unfortunate misstep? (I’m still a fan of STATnews, despite the jab here)
Overall, the news that IPS cells have been safely transplanted into an individual with Parkinson’s does represent a step forward for the community and paves the way for further investigations of this nature. Larger studies, with longer term follow ups are now needed to determine how successful this approach will ultimately be.

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The author of this post is an employee of the Cure Parkinson’s Trust which supports the G-FORCE consortium. The Trust has not asked for this post to be written. This post has been written by the author solely for the purpose of sharing what the author considers interesting information.
The banner for today’s post was sourced from Youtube




Why would the patient continue to need such a high dose of levodopa if the surgery worked well. Also, the journal claims that the pet improvements were modest at best. I’m not sure what to make of these statements. Is this the placebo effect at work, Simon?
LikeLike
There has not been a significant improvement in the UPDRS either
LikeLike
At the World Parkinson’s Congress in Kyoto Dr. Yamanaka discussed strategies for future iPS research during the FSL Special Lecture on June 7, 2019. Is there any chance that you could post a link to that lecture? Thanks for all you do. From the perspective of Covid-19 stay-home isolation SoPD feels like a lifeline connection to PD research.
Dan McEachin
Troy, MI, USA
LikeLiked by 1 person
In my opinion the second stat news article was chasing clicks. The first got picked up by some major outlets in the us. The follow up did too…. The comments on the second were great. They very much thrashed the article till they got locked down.
Thanks for the post Simon!
LikeLike
The numbers look unconvincing no?
LikeLike
It seems to me that a major tradeoff for completely avoiding immunosuppressants is greatly increased cost.
Here is a quote from a 2018 PNT article that discussed Jeanne Loring’s work with autologous iPS cells:
”The biggest downside to this technology is its cost, which is still fairly high, but Loring says the economic burden would not be higher in comparison with other personalized cell therapies, such as CAR-T cell therapies — priced at roughly $500,000.”
https://parkinsonsnewstoday.com/2018/07/26/stem-cells-promising-parkinsons-therapy-special-issue/
In contrast to this, here is a quote from a 2018 Mainichi article that discussed Jun Takahashi’s work with allogeneic iPS cells:
“The researchers are aiming to develop the method as a new treatment covered by national health insurance.”
LikeLike
I am a scientist in the field and work on neurodegenerative disorders. I think the biggest black hole in this set of research is the assumption that the only thing worth considering in PD is dopaminergic neurons. I know that sounds crazy but here me out. Dopamine neurons are incredibly important, with many functions in the CNS, the best described in terms of movement and communication within the nuclei of the basal ganglia. This issue I have is that these neurons are not just degenerating on their own, there is an entire environment which is changing around them. The changing reactivity of glial cells, increases in oxidative stress, defective endosomal-lysosomal trafficking, dysfunctional recycling and protein degradation, RNS accumulation, defective brain glucose metabolism. Furthermore, how are oligodendrocytes, astrocytes and microglia changing not only in the ageing brain but specifically in the brains of people with sporadic PD? Until we can answer those questions and identify these defined changes, it is not appropriate to attempt stem cell therapy in this way. In my opinion, introducing ‘new’ dopamine neuron stem cells into a pathological environment is not going to work. It may be promising as an asymptomatic treatment however.
The final thing I would say regarding the funding of this work is that I completely understand and agree with the ethical issues raised. However, this work in the context of both its efficacy in treating PD and it’s use in the general population is very much in embryo. It could also be considered as positive that this work, which would have cost a lot of money but only showed very minor positive effects, was funded privately.
LikeLiked by 1 person
“It may be promising as an asymptomatic treatment however”. Can you elaborate on this?
LikeLike
I would still think gene therapy approach is a much better approach with the much higher chance of efficacy and lower dyskinesia. If dopamine is the need you can titrate it with longer acting oral meds, which are expected to restore levodopa response to what it was in early stage disease, a wonder drug. If you have increased amount of on time with drugs, lower and infrequent doses that should do the trick from a motor standpoint. While the stem cell therapies are still in the works, there are a lot of unknowns. It has to be stated that two gene therapies for Parkinson’s for dopamine management are already in the clinic, with Voyager’s AADC therapy given fast track designation by the FDA based on how promising the results were. there’s a lot of unknowns here with the stem cell. I wish them well but I’m only going to be very cautiously optimistic..
I’m just an engineer that has spoken to some of the exports in this field. These thoughts are combination of theirs and mine
LikeLike