|
# # # # Last year the results of the large STEADY-PD study were published. The investigators behind the Phase III clinical trial reported that the experimental treatment being tested had no effect on the progression of Parkinson’s in recently diagnosed individuals. The treatment being evaluated was a calcium channel blocker called isradipine – it is used for treating high blood pressure. Since publishing the results, some of the researchers behind the study have been conducting post hoc analysis of the data… and they have found something interesting: An effect. In today’s post, we will look at why isradipine was evaluated in Parkinson’s, what the results of the STEADY-PD study were, and what the newly discovered effect could mean. # # # # |
 Source: Medium
Source: Medium
In the scientific world, post hoc analysis (from the Latin post hoc, meaning “after this”) consists of statistical analyses that are specified after the data has been seen. This type of analysis should only be considered for “hypothesis forming” exercises, and not be viewed as cherry-picking of the data in order to find an effect.
And one must be careful with interpretation of data (eg. most people who are involved in car crashes have been reported as wearing clothes at the time of the incident, thus we should get rid of clothes to assess if this will reduce the incidence of automobile accidents).
Post hoc analyses of completed clinical trial data, however, can be very useful process of identifying interesting trends that could be explored in future studies.
A good example of this has recently been conducted on the STEADY-PD clinical trial study.
What was the STEADY-PD clinical trial about?
STEADY–PD (Safety, Tolerability and Efficacy Assessment of Dynacirc CR in Parkinson Disease) was a series of clinical trials evaluating the calcium channel blocker isradipine (brand named Dynacirc CR) in people recently diagnosed with Parkinson’s.
 Isradipine. Source: Dailymed
Isradipine. Source: Dailymed
Why were they testing this drug?
Isradipine is a calcium channel blocker of the dihydropyridine class.
It is generally prescribed for the treatment of high blood pressure in order to reduce the risk of stroke and heart attack. Prof Frank Church over at the ‘Journey with Parkinson’s’ blog has a great page on isradipine (Click here to read that post).
Why was a calcium channel blocker being tested on Parkinson’s?
There is a grand theory of Parkinson’s based around the chemical element calcium (aka Ca2). It goes something like this:
Dopamine neurons (the cells that are particularly vulnerable in brain of a person with Parkinson’s) have a distinct property: They are autonomously active. That is to say, they are continuously generating a low frequency of activity, even in the absence of any direct input.
This ‘pacemaker’-like activity is believed to be responsible for the sustained release of dopamine in a region of the brain called the striatum (the caudate nucleus and putamen in the image below). This sustained release is considered to be necessary for proper functioning in this region of the brain.
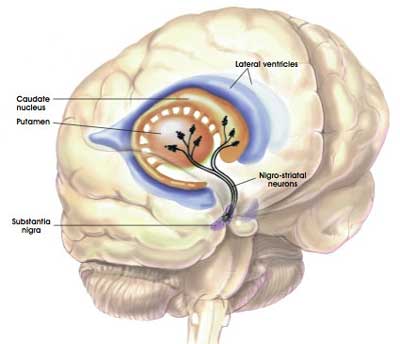
The projections of the dopamine neurons up into the striatum. Source: MyBrainNotes
This spontaneous electrical ‘pacemaker’ activity is believed to be dependent on calcium channels – particularly L-type calcium channels. If you block these calcium channels, the pacemaker property of dopamine neurons disappears.
Now please have a look at the image below:
 A calcium channel. Source: Wikipedia
A calcium channel. Source: Wikipedia
In the image above, there is a representation of a calcium channel embedded in the cell membrane. In the top left side of the image are three word written in red (Benzothiazepine, Phenylalkylamines & Dihydropyridines). These are inhibitors of calcium channels. They represent a class of medication called calcium channel blockers, which are used to treat hypertension (or long-term high blood pressure).
Ten years ago, researchers noticed something interesting about calcium channel blockers that specifically target the L-type calcium channels, and they published their results in this report:
 Title: L-type calcium channel blockers and Parkinson disease in Denmark.
Title: L-type calcium channel blockers and Parkinson disease in Denmark.
Authors: Ritz B, Rhodes SL, Qian L, Schernhammer E, Olsen JH, Friis S.
Journal: Ann Neurol. 2010 May;67(5):600-6.
PMID: 20437557 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers collected the medical records of 1,931 people in Denmark who were diagnosed with Parkinson’s between 2001 and 2006. These records were age and sex matched to 9,651 records of healthy controls from the same register. After analysing all of the records, the investigators found that people prescribed with a type of medication called a L-type calcium channel blocker (dihydropyridines) were 27% less likely to develop Parkinson’s.
This finding supported a previous study that found a similar result (Click here to read more about that) and the same result was independently replicated a couple of years later (Click here to read that report).
And the result was further supported by postmortem analysis of the Parkinsonian brain:
 Title: Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins.
Title: Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins.
Authors: Hurley MJ, Brandon B, Gentleman SM, Dexter DT.
Journal: Brain. 2013 Jul;136(Pt 7):2077-97.
PMID: 23771339 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers looked at where in the human brain the L-type calcium channels were present and in what concentration. They used sections of postmortem brain to carry out this analysis, looking at both healthy specimens as well as those from people who passed away with Parkinson’s.
In the normal brains, the distribution of the L-type calcium channels and certain calcium-binding proteins was not associated with regions of the brain that are prone to the selective neurodegeneration that is seen in Parkinson’s.
But the investigators observed a very different picture in the Parkinsonian brains. Increased levels of L-type calcium channels and the calcium-binding proteins were found throughout the brains of people who passed away with Parkinson’s. Even in cases of early Parkinson’s (in people who passed away shortly after being diagnosed), increased levels of L-type calcium channels were even found in the cerebral cortex – a part of the brain largely unaffected in Parkinson’s.
These findings lead the researchers to conclude that “disturbed calcium homeostasis” may be “an early feature of Parkinson’s disease and not just a compensatory consequence to the neurodegenerative process”.
But how could calcium possibly be involved with the mechanisms underlying the neurodegeneration seen in Parkinson’s?
There are actually numerous ways that calcium could be playing a role – from mitochondrial function to oxidative stress (Click here and here to read good reviews on this topic).
There have also been a number of research reports demonstrating interactions between calcium and the bad boy of Parkinson’s research: a protein called alpha synuclein, which clumps together in the brain of people with Parkinson’s.
One example of such research is this study:
 Title: C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction.
Title: C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction.
Authors: Lautenschläger J, Stephens AD, Fusco G, Ströhl F, Curry N, Zacharopoulou M, Michel CH, Laine R, Nespovitaya N, Fantham M, Pinotsi D, Zago W, Fraser P, Tandon A, St George-Hyslop P, Rees E, Phillips JJ, De Simone A, Kaminski CF, Schierle GSK.
Journal: Nat Commun. 2018 Feb 19;9(1):712.
PMID: 29459792 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers wanted to look at how calcium could be affecting the normal functioning of alpha synuclein.
Using a special technique (called ‘Chemical exchange saturation transfer-nuclear magnetic resonance‘ – please don’t ask me what that means), the researchers were able to watch what happens to the protein and calcium when they encounter each other.
When the researchers compared the binding of different ions to alpha synuclein, they found that calcium in particular had a strong affinity for alpha synuclein:
 Source: PMC
Source: PMC
Given that their results, the researchers next asked whether this process could be involved with aspects of Parkinson’s. The investigators found that the combination of calcium and dopamine promoted the clustering (or aggregation) of alpha synuclein. Interestingly, the researchers found that these dopamine-induced changes could be reversed back to normal by treating the cells with the calcium channel blocker, isradipine (remember this drug?).
 Source: PMC
Source: PMC
The researchers also noticed that the addition of dopamine to the combination of calcium and alpha synuclein appeared to cause some toxicity effect resulting in cell death in the experiment. So they next looked at whether the removal of calcium (using the calcium channel blocker isradipine) could reduce this cell loss.
And they found that it did:
 Source: PMC
Source: PMC
There is a lot of additional independent data demonstrating that levels of calcium within a cell (in addition to oxidative stress) can promote alpha synuclein protein aggregation (Click here to read a review on this topic).
Thus, there was ample evidence to justify a clinical trial testing if reducing calcium levels inside cells can help to slow the progression of Parkinson’s.
|
# RECAP #1: Accumulating evidence supports the idea that the chemical element calcium is potentially playing a role in Parkinson’s. Nation-wide, longitudinal data suggests that use of certain calcium channel blocking medication may reduce the risk of developing Parkinson’s. In addition, calcium channel blockers (like isradipine) reduce toxicity in models of Parkinson’s. # |
So what happened in the clinical trials?
The STEADY-PD program started with a pilot study – the results of which were published in 2010:
 Title: Tolerability of isradipine in early Parkinson’s disease: a pilot dose escalation study.
Title: Tolerability of isradipine in early Parkinson’s disease: a pilot dose escalation study.
Authors: Simuni T, Borushko E, Avram MJ, Miskevics S, Martel A, Zadikoff C, Videnovic A, Weaver FM, Williams K, Surmeier DJ.
Journal: Mov Disord. 2010 Dec 15;25(16):2863-6.
PMID: 20818667
In this study, the researchers wanted to assess the tolerability of isradipine in 31 individuals with recently diagnosed Parkinson’s (not requiring any dopaminergic therapy). The investigators gave participants increasing doses of the drug (from 5 to 20 mg per day) over the course of 8 weeks. The vast majority had no problems with the low dose, but almost half had issues tolerating the highest dose.
This study gave the researchers confidence that the drug was safe in people with Parkinson’s as they planned a larger and longer study to assess the long term safety of isradipine.
The results of that larger study were published in 2013:
 Title: Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD).
Title: Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD).
Authors: Parkinson Study Group.
Journal: Mov Disord. 2013 Nov;28(13):1823-31.
PMID: 24123224
In this Phase 2, randomised, double-blind, parallel group study, the researcher enrolled 99 subjects to take 3 different doses (5, 10, or 20 mg) of isradipine or a matching placebo everyday for 52 weeks. The goal of the study was to assess safety and tolerability over a long period of time, and identify the best dose for a larger Phase 3 trial.
The researchers found that the tolerability of isradipine was dose dependent (this means that as the dose went up, the treatment was less well tolerated. In the placebo group, 25 of the 26 participants (96%) found the treatment tolerable, and this was comparable to the 5 mg group, where 19 of 23 (83%) participants considered the tolerable. But in the 10 mg group, 19 of 26 participants (73%) thought that the treatment was easily tolerated, and this dropped significantly in the 20 mg group, where only 9 of 24 participants (37%) found the treatment tolerable.
The investigators observed no difference in change of clinical scores (as determined by the Unified Parkinson’s Disease Rating Scale or UPDRS) across any of the groups, but the study helped to identify the maximal tolerable dose of 10 mg daily for the follow up Phase 3 trial.
So what happened with the Phase 3 study?
The double blind, Phase III STEADY-PD clinical trial was conducted at 56 research centres across the US & Canada, enrolling over 300 people with newly diagnosed Parkinson’s to take either isradipine (5 mg of immediate-release isradipine twice daily) or placebo for 36 months (participants were randomly assigned to one of the two groups – click here and here to read more about the clinical study).
Recruitment started in November 2014 and the last participant completed the 3-year treatment regime in November 2018.
On the 30th March 2019, the investigators coordinating the trial issued a press release that informed the PD community that the STEADY-PD Phase 3 trial had not met its primary endpoint – the endpoint being the predetermined measure that would determine efficacy. There was no difference between in clinical progression of Parkinson’s between the group treated with isradipine or the placebo group.
The results of the study were published in 2020:
 Title: Isradipine Versus Placebo in Early Parkinson Disease: A Randomized Trial.
Title: Isradipine Versus Placebo in Early Parkinson Disease: A Randomized Trial.
Authors: Parkinson Study Group STEADY-PD III Investigators.
Journal: Ann Intern Med. 2020 May 5;172(9):591-598.
PMID: 32227247 (This report is OPEN ACCESS if you would like to read it)
The results of the study suggest that the two groups of participants were evenly randomised (170 in the isradipine group vs 166 in the placebo group). The average ages were 62.1 and 61.6 yrs, respectively, and the stage of Parkinson’s at baseline were also alike (UPDRS scores of 23.7 vs 22.6, respectively). The two groups appear to have been well balanced.
At the end of the study though, there was no difference between the two groups. The report shows very similar rates of progression in both the isradipine and placebo groups.
In their concluding statement, the researchers questioned whether the dose used in the study was high enough to have a large enough effect on calcium levels. But they acknowledge that this was the preferred tolerable dose (based on the Phase 2 study). They also queried if post diagnosis was the wrong time to be administering the drug. Given that the epidemiological studies (the nationwide, longitudinal research discussed above) shows a reduced risk of developing Parkinson’s, perhaps isradipine would be better administered to individuals at risk of developing Parkinson’s (for example, those exhibiting symptoms of the prodromal phase of Parkinson’s).
What is the prodromal phase of Parkinson’s?
The ‘prodromal phase’ of the condition is the period of time before diagnosis. It involves some of the very early signs of the Parkinson’s (such as constipation, loss of a sense of smell, and REM sleep disorder – more on this in a moment), and the very first signs of motor/movement problems (a twitching finger, or a dragging leg).
 Source: Guidelinesinpractice
Source: Guidelinesinpractice
The investigators concluded their report by saying “that treatment with 5 mg of immediate release isradipine twice daily did not slow the clinical progression of early-stage PD, as measured by the change in the UPDRS score and various other measures and instruments”.
|
# # RECAP #2: The STEADY-PD clinical trial program was initiated to evaluate the calcium channel blocker isradipine as a disease modifying therapy for Parkinson’s. The results of the Phase III clinical trial – involving 300 people with recently diagnosed Parkinson’s – indicated that the drug had no effect on the progression of the condition. # # |
So what have the researchers discovered in their post hoc analysis of the STEADY-PD clinical trial data?
In January of this year, this report was published:
 Title: Isradipine plasma pharmacokinetics and exposure-response in early Parkinson’s disease.
Title: Isradipine plasma pharmacokinetics and exposure-response in early Parkinson’s disease.
Journal: Venuto CS, Yang L, Javidnia M, Oakes D, James Surmeier D, Simuni T.
Journal: Ann Clin Transl Neurol. 2021 Jan 18. Online ahead of print.
PMID: 33460320 (This report is OPEN ACCESS if you would like to read it)
In this study, the investigators were interested in the inter-individual variability associated with isradipine pharmacokinetics, and they thought that an analysis of this could presented the opportunity to explore potential exposure–response relations.
Que?!? What does any of that mean???
Ok, let’s start with “inter-individual variability associated with isradipine pharmacokinetics”.
When each of us takes a pill, there will be differences between us in how fast the pill is broken down in our guts and then absorbed into our bodies. This is the “interindividual variability” part of the sentence.
And what about pharmacokinetics? What does that mean?
In clinical trials, researchers are interested in investigating the pharmacodynamics and pharmacokinetics of a treatment.
Pharmacodynamics explores how a drug affects an organism (eg. mechanism of action) – basically, what does the drug do to the body?
Pharmacokinetics, on the other hand, looks at how the organism affects the drug (eg. how well is it absorbed and excreted) – basically, what does the body do to the drug.
 Source: Pinterest
Source: Pinterest
Pharmacokinetics can vary from person to person based on many different variables, from genetics to environmental factors.
Ok, so they wanted to look at the differences between how people’s bodies were dealing with the drug. But what was the “opportunity to explore potential exposure–response relations” part about?
The researchers simply wanted to determine if the variability in pharmacokinetics could be masking any potential effects of the drug. For example, was the drug being broken down and excreted too quickly in too many people in the study for it to be seen to have a disease halting effect?
In particular, the scientists were interested in differences in isradipine exposure. That is to say, did the participants with the highest levels of the drug experience any effect?
Got it. So how did they look at this?
The investigators analysed the blood samples collected from nearly all of the study participants who were randomised to the isradipine (5‐mg twice daily) group (samples from 166 of the 170 participants).
In their results, the researchers found that different levels of isradipine exposures did not impact the primary clinical outcome of the study (changes in Unified Parkinson’s Disease Rating Scale parts I–III score).
But when they looked at when participants needed to initiate anti-Parkinson’s treatment (such as L-dopa) over the 36 months study, they found that higher isradipine exposure was associated with a delay in the need to initiate anti-Parkinson’s treatments and a lower dose of L-dopa at the start of that treatment.
The researchers concluded their study by stating: “While these results raise the possibility that the study failed because of insufficient drug exposures, dose‐limiting side effects deterred increasing isradipine dose with the IR formulation”
And they added that “more targeted and sustained delivery of L‐type calcium channel inhibitors to the brain would provide a better test of their disease‐modifying potential”.
And this last statement is interesting given recent preclinical data.
What preclinical data?
In 2019, this report was published:
 Title: Genetic silencing of striatal CaV1.3 prevents and ameliorates levodopa dyskinesia
Title: Genetic silencing of striatal CaV1.3 prevents and ameliorates levodopa dyskinesia
Authors: Steece-Collier K, Stancati JA, Collier NJ, Sandoval IM, Mercado NM, Sortwell CE, Collier TJ, Manfredsson FP.
Journal: Mov Disord. 2019 May;34(5):697-707.
PMID: 31002755 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers were interested in reducing already established dyskinesias.
What are dyskinesias?
Dyskinesias (from Greek: dys – abnormal; and kinēsis – motion, movement) are a category of movement disorders that are characterised by involuntary muscle movements. And they are certainly not specific to Parkinson’s.
But in the case of Parkinson’s, dyskinesias have generally been believed to be associated with long-term use of Levodopa (also known as Sinemet or Madopar).

Sinemet is Levodopa. Source: Drugs
NOTE: Long-term use of Levodopa is not a certainty for developing dyskinesias, but there is an association. It will differ from person to person.
We have previously discussed dyskinesias on the SoPD (Click here to read more about this topic).
The researchers decided to explore the use of gene therapy to prevent the development of Levodopa-induced dyskinesias.
What is gene therapy?
Gene therapy is an experimental treatment approach that involves treating medical conditions with DNA rather than drugs.
Gene therapy basically involves introducing a new piece of DNA or replacing a faulty piece of DNA within a population of cells. DNA, as you may remember from high school science class provides the instructions for making proteins in the a cell and these proteins are the bits that actually do stuff.

Gene therapy for Parkinson’s disease. Source: Wiki.Epfl
The researchers started their experiment by injecting rats with the gene therapy that would reduce levels of the calcium channel CaV1.3. The virus was injected into a region of the brain called the striatum, which is where dopamine neurons release much of their dopamine.
Next they induced a model of Parkinson’s using a neurotoxin (6-OHDA) and then they started treating the animals with high doses of Levodopa to cause Levodopa-induced dyskinesias (see panel A in the image below for a schematic of this study design).
 Source: Wiley
Source: Wiley
The researchers found that rats who were injected the CaV1.3 gene therapy did not develop dyskinensias… at all!
If you look at panel B in the image above, you can see a black line of the control rats (no gene therapy, but model of Parkinson’s with Levodopa-induced dyskinesias) rapidly develop the dyskinesias with increasing severity as the dose of Levodopa is increased. The red line bouncing along the bottom of the graph are the gene therapy treated animals with no dyskinesias.
This was a really impressive result.
But the researchers wanted to take this finding one step further and see if they could stop established dyskinesias – that is to say, can the gene therapy approach work after dyskinesias have already developed?
So the investigators repeated the experiment, except this time they induced a model of Parkinson’s using a neurotoxin (6-OHDA) and treating the animals with high doses of Levodopa to cause Levodopa-induced dyskinesias before they treated some of the animals with the gene therapy treatment (see panel A in the image below for a schematic of this study design).
As you can see from panel B of the image below, the red line of the gene therapy treated animals starts to reduce from about 2 weeks post-gene therapy treatment. The gene therapy treatment resulted in a significant reversal of established, severe Levodopa induced dyskinesias.
 Source: Wiley
Source: Wiley
And remarkably, the researchers demonstrated that this CaV1.3 reducing gene therapy did not interfere with the normal motor response to Levodopa. That is to say, Levodopa retained its ability to improve motor function in the rodent models of Parkinson’s despite the gene therapy treatment.
And this research comes back to the statement made by the STEADY-PD investigators regarding “more targeted and sustained delivery of L‐type calcium channel inhibitors to the brain”. Isradipine targets CaV1.3, but in order for a therapy to be more tolerable, perhaps a more targeted delivery (like gene therapy) is required.
It will be interesting to see which direction the researchers take the line of investigation next.
So what does it all mean?
It is important to remember that post-hoc analysis is only a hypothesis-generating tool. It can not be used to identify “effects” in the results of a study. If a study was not designed to measure a specific effect, then anything else should simply be considered an association and be explored in subsequent studies.
Recently researchers involved in the STEADY-PD clinical trial program have published post-hoc analysis of their Phase III clinical trial data that indicates that participants with higher exposure to the treatment drug (isradipine) had a delayed time to the initiation of Levodopa treatment and started on lower doses.
Whether this represents a “disease modifying” effect still needs to be explored in carefully designed experiments, which require close monitoring given the treatment tolerable issues with high doses of the drug. Ongoing preclinical research is also exploring more targeted approaches towards modulating calcium channels in the appropriate parts of the brain, potentially circumventing the dose tolerable issues.
It would be interesting to see if the Phase I and Phase II data from the STEADY-PD program also demonstrate this effect. Such data would provide strong justification for further exploration of this work.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from

Fascinating – thank you. A brilliant explanation. I was prescribed Isradipine for hypertension for 18 months post PD diagnosis but not available on NHS and difficult to obtain so switched to Felodipine. Seems there is nothing to lose and potentially lots to gain……
LikeLike