This is Tom Isaacs. He is the charismatic founder of the Cure Parkinson’s trust.

Tom Isaacs. Source: GrannyButtons
He’s a dude.
The man walked the entire coastline of the UK to raise money/awareness for Parkinson’s disease! Trust me, he’s a dude.
The title of today’s post is a salute to Tom’s efforts to offer a humourous label to what is a very serious and debilitating aspect of Parkinson’s disease.
In this post, we will discuss the science of dyskinesias
For the last 50 years, Levodopa (L-dopa) has been the “gold standard” treatment for Parkinson’s disease. By replacing the lost dopamine, L-dopa allows for the locomotion parts of the brain to become less inhibited and for people with Parkinson’s disease to feel more in control of their movements.
This miraculous treatment, however, comes at a terrible cost.
After long-term use of the drug, abnormal and involuntary movements can begin to appear. These movements are called dyskinesias.

An example of a person with dyskinesia. Source: JAMA Neurology
What are Dyskinesias?
Dyskinesias (from Greek: dys/dus – difficulty, abnormal; and kinēsis – motion, movement) are simply a category of movement disorders that are characterized by involuntary muscle movements. They are certainly not specific to just Parkinson’s disease.
In Parkinson’s disease, they are associated with long-term use of L-dopa.
An example of dyskinesia can be seen in this video of Tom Isaacs and David Sangster discussing life with Parkinson’s disease (Tom was diagnosed at age 26 years of age and has lived with Parkinson’s for 20 years – he has dyskinesias. David was diagnosed in 2011 at age 29; since diagnosis he foundered www.1in20Parkinsons.org.uk. He’s also a dude!).
How do dyskinesias develop in Parkinson’s disease?
Before beginning a course of L-dopa, the locomotion parts of the brain in people with Parkinson’s disease is pretty inhibited. This results in the slowness and difficulty in initiating movement. They want to move, but they can’t. They are akinetic.
L-dopa tablets provide the brain with the precursor to the chemical dopamine. Dopamine producing cells are lost in Parkinson’s disease, so replacing the missing dopamine is one way to treat the motor features of the condition. Simply giving people pills of dopamine is a non-starter: dopamine is unstable, breaks down too quickly, and (strangely) has a very hard time getting into the brain. L-dopa, on the other hand, is very robust and has no problem getting into the brain.
Once inside the brain, L-dopa is quickly converted – via an enzymatic reaction – into dopamine allowing the locomotion parts of the brain to function close to normal. In understanding this process, it is important to appreciate that when a tablet is taken and L-dopa enters the brain, there is a sudden rush of dopamine. A spike in it’s supply, and for the next few hours this gradually wears off as the dopamine is used up. This tablet approach to L-dopa treatment gives a wave like shape to the L-dopa levels in the brain over the course of the day (see the figure below).
After prolonged use of L-dopa (7-10 years on average), the majority of people with Parkinson’s disease will experience a shorter response to each dose of L-dopa. They will also find that they have more time during which they will be unable to move (exhibiting akinesia). This is simply the result of the disease progression – L-dopa treats the motor features of the disease but hides the fact that the disease is still progressing.
This shortening of response is often associated with subtle abnormal involuntary movements that appear when the levels of l-DOPA are highest (usually soon after taking a tablet). It is as if there is too much dopamine for the system to handle.
Gradually, the response time (during which normal movement is possible) will grow shorter and to combat this the dose of L-dopa is increased. But with increased levels of L-dopa, there is an increase in the involuntary movements, or dyskinesias.

This figure illustrates the course of Parkinson’s disease for some people on L-dopa. The waving line indicates the level of L-dopa in the blood (as a result of taking L-dopa medication). The white space is the area where normal movement is possible, while the grey area illustrates periods of akinesia (inability to move). Without L-dopa, people with Parkinson’s disease would be stuck in this area, and as the L-dopa pill wears off (during the downward part of the waving line) they fall back into the akinesia area, thus requiring another pill. As the disease progresses, the akinetic (grey) area increases, requiring higher levels of L-dopa to be administered in order to escape it. The tan coloured area in the top right corner demonstrates the introduction of dyskinesias.
Are there different types of dyskinesias?
Yes there are. Dyskinesias have been broken down into many different subtypes, but the two main types of dyskinesia are:
Chorea – these are involuntary, irregular, purposeless, and unsustained movements. To an observer, Chorea will look like a very disorganised/uncoordinated attempt at dancing (hence the name, from the Greek word ‘χορεία’ which means ‘dance’). While the overall activity of the body can appear continuous, the individual movements are brief, infrequent and isolated. Chorea can cause problems with maintaining a sustained muscle contraction, which may result in affected people dropping things or even falling over.
Dystonia – these are sustained muscle contractions. They often occur at rest and can be either focal or generalized. Focal dystonias are involuntary contractions in a single body part, for example the upper facial area. Generalized dystonia, as the name suggests, are contraction affecting multiple body regions at the same time, typically the trunk, one or both legs, and another body part. The intensity of muscular movements in sufferers can fluctuate, and symptoms usually worsen during periods of fatigue or stress.
When were Dyskinesias first discovered?
Ironically but unsurprisingly, L-dopa induced dyskinesias were first reported by the same scientists who first reported the drug’s amazing effects in Parkinson’s disease:

Title: Modification of Parkinsonism – chronic treatment with L-dopa.
Authors: Cotzias GC, Papavasiliou PS, Gellene R.
Journal: New England Journal of Medicine. 1969 Feb 13;280(7):337-45.
PMID: 4178641
George Cotzias was one of the first physicians to give L-dopa to people with Parkinson’s disease.

Dr George Cotzias. Source: NewScientist
Cotzias and colleagues administered L-dopa to 28 people with Parkinson’s disease (17 males and 11 females) and observed modest to moderate response in 8 of them, a marked response in 10, and dramatic responses in the other 10 people. During their two year observation period, they also reported seeing involuntary movements (dyskinesias) in half of the subjects in the study (14/28). They ranged from rare and fleeting (eg. grimacing or gnawing and wave-like motions of the head) to severe (eg. full body/limb movements). They noted that the dyskinesias were most severe in the people with the longest duration of the disease.
It should be noted that the speed with which some of the patients (that Cotzias was treating) developed their dyskinesias – less than 2 years – was a reflection on the late stage of the condition at which the treatment was begun. When the administration of L-dopa is started at an earlier stage, the window of effective treatment is generally longer (5-10 years, depending on individual cases).
So what causes the dyskinesias?
Oh boy.
This question is the source of much debate.
Volumes of text have been bashed out and sides have been taken. We are going to have to tread very carefully here for fear of upsetting folks is the world of Parkinson’s research.
There is some agreement, however, that the factors associated with the development of L-dopa-induced dyskinesias include:
- the duration of the disease
- the severity of the disease
- the dose of L-dopa (cue the debating)
- young age onset
There are also some genetic forms of Parkinson’s disease that can have an impact on the chances of developing dyskinesias.
Duration/severity of the disease – Experimental studies in animal models of Parkinson’s disease indicate that, if L-dopa is given to the animals, involuntary movements will only develop when the loss of dopamine in the brain exceeds 80–85% of normal. Clinical observations, however, indicate that the severe loss of dopamine in the brain is not sufficient for patients to develop dyskinesias.
This has lead to theories regarding intact part of the brain, suggesting that there are changes in the neurons that the dopamine is acting on. And indeed postmortem analysis of brains from people with & without dyskinesias suggests that there are differences in the neurons that dopamine act on (Click here and here for more on this).
The dose of L-dopa – in a large clinical study, the researchers found that an average daily L-dopa dose of 338 mg was not associated with dyskinesias, while an average daily dose of 387 mg was (Click here and here to read more on this).
Young age onset – Given the length of time that people with early-onset Parkinson’s disease will be on L-dopa, there is a strong association between early-onset and dyskinesias.
EDITORIAL NOTE: We are now about to discuss what can be done to alleviate dyskinesias. Before doing so, we here at the Science of Parkinson’s disease would just like to repeat our standard warning that the contents provided on this website is of an informative nature, and no actions should be taken based on what you have read without first consulting your doctor. Please seek medical advice before changing or experimenting with your treatment regime.
And what can be done to alleviate dyskinesias?
There are several methods of reducing dyskinesias:
Reducing L-dopa dose
Obviously, one can lower the dose of L-dopa. This almost always results in a reduction of dyskinesias. BUT, this almost always results in a worsening of Parkinson’s disease motor features, so it can’t really be considered a solution.
Dopamine receptor agonists
Rather than giving the brain L-dopa or dopamine, chemicals that behave exactly like dopamine can be administered. Dopamine receptor agonists are drugs that act on the receptors of dopamine that are present on the cells that dopamine acts on. These drugs have a longer half‐life than levodopa, meaning that they hang around in the brain for longer (eg. they are not broken down or used up as quickly as L-dopa).
In a large double‐blind study that compared the safety and efficacy of a dopamine receptor agonist – ‘Ropinirole’ – with that of levodopa over a period of five years, researchers found that the incidence of dyskinesia (regardless of levodopa supplementation) was 20% in the ropinirole group and 45% in the levodopa-only group (Click here for more on that study, and click here for a similar study with the dopamine agonist pramipexole).
One cautionary note – Dopamine agonists have been associated with the development of compulsive and impulsive behaviours (Click here for more on this).
Drugs acting on NMDA receptors
N-methyl-D-aspartate receptors (NMDA receptors) are receptors of the chemical glutamate. They are widely found in the brain, but during dyskinesias they appear to become more abundant. As a result, researchers have used drugs that block NMDA receptors (called NMDA receptor antagonists) as potential treatment for dyskinesias. And they appear to help in many cases.
In a double‐blind, placebo‐controlled study of 18 people with Parkinson’s disease, researchers found that the NMDA receptor antagonist ‘Amantidine’ reduced the duration of L-dopa-induced dyskinesias by 60% (Click here for more on this).
Drugs acting on serotonergic systems
Recently there has been a lot of attention focused on the role in dyskinesias of another chemical in the brain: serotonin. There is significant loss of serotonergic cells and fibres in the brain of people with Parkinson’s disease, though not to the same scale as dopamine.
A recent clinical study investigating the use of drugs that prolong the serotonin floating around in the brain (called selective serotonin reuptake inhibitors or SSRIs), found that they did not protect people with Parkinson’s disease from dyskinesias, but may delay their onset (Click here for more on this). There are also clinical trials investigating the use of serotonin receptor agonists in Parkinson’s disease with dyskinesias, based on positive results from preclinical studies (Click here for more on this).
More recently researchers have been investigating the role of serotonin cells in the production of dopamine from L-dopa. Serotonin cells are known to absorb L-dopa and to convert it into dopamine, but they do not have any means of storing it and they release it in an uncontrolled fashion. Recent studies in rodent models of L-dopa-induced dyskinesias have reported reductions in dyskinetic behaviour as a result of lesioning the serotonin cells or blocking specific serotonin receptors. The clinical relevance of these finding is yet to be tested, however.
Neurosurgery
The use of ‘pacemaker’ surgeries (such as deep brain stimulation targeting regions such as the globus pallidum or subthalamic nucleus) have been shown to be effective in treating advanced Parkinson’s disease. The resulting motor improvements are also associated with a reduction in dyskinesias.
A blinded pilot study comparing the safety and efficacy of deep brain stimulation in people with advanced Parkinson’s disease reported a 60-90% reduction in dyskinesias, depending on the region of the brain that was targeted (Click here for more on this).
Surgical lesions targeting the thalamus, globus pallidum or subthalamic nucleus have also been used in the treatment of advanced Parkinson’s disease, with reductions in dyskinesias also being observed. It is effective in both young as well as elderly subjects, with benefit persisting for up to 5 years. These surgical lesion procedures, however, are irreversible.
Recent advances in our understanding
We always like to bring you new research here at the Science of Parkinson’s disease and recently there have been some interesting results published. For example, this one:

Title: Serotonin-to-dopamine transporter ratios in Parkinson disease: Relevance for dyskinesias.
Authors: Roussakis AA, Politis M, Towey D, Piccini P.
Journal: Neurology. 2016 Published Feb 26.
PMID: 26920358
The researchers in this study conducted brain imaging on people with Parkinson’s disease who did have dyskinesias (17 people) and did not have dyskinesias (11 people). Specifically they were looking to see the difference in the density of dopamine and serotonin fibres in particular areas of the brain affected by dyskinesias. They found that people with Parkinson’s disease AND dyskinesias had a higher ratio of serotonin fibres to dopamine fibres than people with Parkinson’s disease but no dyskinesias. This result adds further support to the role that serotonin cells are playing in the development of L-dopa-induced dyskinesias.
Phew, long post.
If you have got this far and you are still reading – thanks! We hope it was informative.
In (shorter) future posts, we will be assessing new research dealing the mechanisms of and novel ways to treat dyskinesias. This post was meant to be an introduction that we will refer back to from time to time.
Stay tuned!






































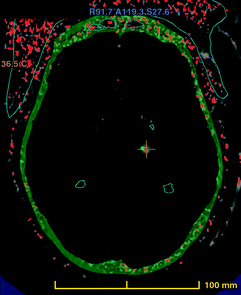 A brain scan image of the area being targeted (red cross). The skull is in green, and the cooled water unit is is red. Source:
A brain scan image of the area being targeted (red cross). The skull is in green, and the cooled water unit is is red. Source: 

