|
# # # # At the end of each month, the SoPD writes a post which provides an overview of some of the major or interesting pieces of Parkinson’s-related research that were made available during February 2026. The post is divided into 10 parts based on the type of research:
# # # # |
So, what happened during February 2026?
In world news:
1st February – In his first ever indoor mile at the Boston University Terriers classic, Kiwi runner Sam Ruthe won in a time of 3:48.88. This was the eleventh fastest time ever recorded indoors, breaking the absolute New Zealand mile record of 3:49.08 of Sir John Walker from 1982, and the New Zealand indoor mile record of 3:51.06 set by Nick Willis in 2016. In 2025, Ruthe became the youngest person to break the four-minute mile. He is only 16 years of age!
6th February – According to data from the European Research Council (ERC), applications from the United States for its starting, consolidator and advanced grants to individual researchers — worth up to €2.5 million (US$3 million) apiece over five years — rose by 120% in its most recent round of calls, compared with an overall rise in applications of 17%. Meanwhile, Advanced Grants — for established principal investigators — saw the greatest leap in US applications, with the number nearly quintupling in just 12 months (Click here to read more about this).
12th February – The Dutch House of Representatives passed the Actual Return in Box 3 Act (Wet werkelijk rendement box 3) – a reform that will tax residents at a flat rate of 36% on unrealised gains (???) earned from savings, stocks, and investments, effective January 1, 2028 (Click here to read more about this).
13th February – The day after being named ‘undisputed champion of beautiful clean coal’ (???), President Trump (and Lee Zeldin – the head of the Environmental Protection Agency – EPA) announced that the EPA has finalized rescinding the endangerment finding. This was the legal basis for regulating greenhouse gas emissions under the US Clean Air Act. When asked what he would tell people concerned about the move, Mr Trump replied, “I’d tell them don’t worry about it, because this has nothing to do with public health. It just was all a scam, a giant scam” (Click here to read more about this)
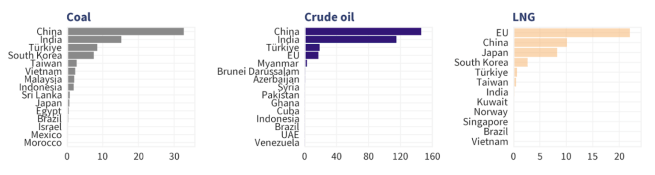
18th February – The Centre for Research on Energy and Clean Air (CREA) January monthly report of Russian fossil fuel exports highlighted that the European Union remains the largest buyer of Russian liquid natural gas (LNG), accounting for almost half (49%) of Russia’s total LNG exports (Click here to read more about this).
In the world of Parkinson s research, a great deal of new research and news was reported:
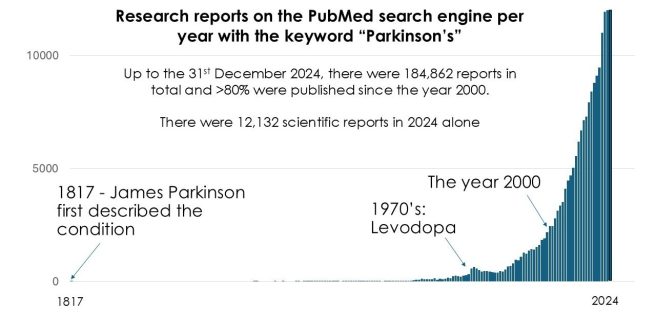
In February 2026, there were 1,391 research articles added to the Pubmed website with the tag word Parkinson s attached (2,803 for all of 2026 so far). In addition, there was a wave to news reports regarding various other bits of Parkinson s research activity (clinical trials, etc).