|
# # # # About 5% of individuals affected by Parkinson’s carry a genetic variation in a region of their DNA called the GBA1 gene. This area of DNA provides the instructions for making an enzyme called GCase, which is known to be involved in cellular waste disposal. Recently, however, researchers have discovered that GCase might have additional functions in cells, particularly in the mitochondria. In today’s post, we will discuss what GCase is, how it is associated with Parkinson’s, and review the new research indicating other roles in cellular biology. # # # # |
 Source: Createvalue
Source: Createvalue
Isn’t it funny how things change.
And I mean, properly change.
Something will start off in life as one thing, and then a new perspective or a conflicting idea is provided and suddenly it becomes something else entirely – changing how we view that thing and its place in the world.
A truly exaggerated example of this is the wonderous story of Adam Rainer.
Born in Austria (1899), Adam was always a small child and by the time he reached 18 years of age he measured just 138 cm (or 4 foot 5 inches). Due to his height, he was technically considered a dwarf (less than 147 cm or 4 foot 10 inches). As result of this designation, he was refused entry into the Austro-Hungarian Army and thus missed out on serving in the First World War.
But then something really strange happened…
From 21 years of age to 32, while everyone else stopped growing, Adam suddenly started to grow.
And when I say grow, I mean grow!
 Adam Rainer (far left). Source: Twitter
Adam Rainer (far left). Source: Twitter
By the time Adam was 32, he had grown to the height of 218 cm (or 7 foot 2 inches). At that time, a benign tumor (a pituitary adenoma) was discovered and removed, slowing Adam’s growth. He died in 1950 at the age of 51, having reached a final height of 234 cm (7 foot 8 inches).
He is the only person in recorded history who started out in life with dwarfism and end it with gigantism (source).
Like I said, a rather exaggerated form of how something can change.
Intriguing, but what does this have to do with Parkinson’s?
Well, today’s post is about how some new data may change our view on an important player in the world of Parkinson’s.
Each week, new scientific research is published. Some of it replicates and confirms previous findings, while other studies refute work published in the past.
But the really exciting stuff is the research that tells us something new about a topic – particularly when it challenges some well established dogma.
And this week we had a good example of this.
What is the new data?
This week, this research report was published:
 Title: Glucocerebrosidase is imported into mitochondria and preserves complex I integrity and energy metabolism.
Title: Glucocerebrosidase is imported into mitochondria and preserves complex I integrity and energy metabolism.
Authors: Baden P, Perez MJ, Raji H, Bertoli F, Kalb S, Illescas M, Spanos F, Giuliano C, Calogero AM, Oldrati M, Hebestreit H, Cappelletti G, Brockmann K, Gasser T, Schapira AHV, Ugalde C, Deleidi M.
Journal: Nat Commun. 2023 Apr 6;14(1):1930.
PMID: 37024507 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers wanted to explore the proteins that interact with the Parkinson’s associated enzyme called glucocerebrosidase (also known as GCase).
What exactly is gluco…cere…bro…sid…ase?
Let’s just use GCase for the rest of this post.
It is an enzyme that helps with the digestion and recycling of waste (particularly glucocerebrosides) inside cells. GCase protein is made using instructions that are in chromosome 1 of our DNA, in a region called the GBA1 gene. Tiny errors (called genetic variants) in the GBA1 gene are associated with an increased risk of developing Parkinson’s.
 Source: 23andMe
Source: 23andMe
The genetic variations in the GBA1 gene are some of the most common genetic risk factors for the condition. It is believed that approximately 5%–8% of people with Parkinson’s have a genetic mutation in their GBA1 gene (Click here and here to read more about this).
What does GCase do? And how could this be affecting Parkinson’s?
Inside of cells, GCase enzyme is produced in an area called the endoplasmic reticulum.
The endoplasmic reticulum (or ER) is a highly convoluted, netlike mesh structure that extends off the nucleus of a cell. It is the factory assembly line where proteins are produced within a cell.
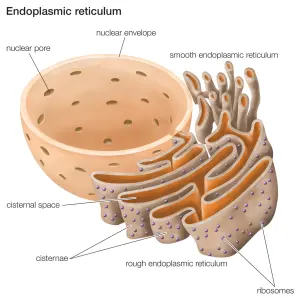 The endoplasmic reticulum connected to the spherical nucleus. Source: Britannica
The endoplasmic reticulum connected to the spherical nucleus. Source: Britannica
The nucleus of the cell is where the blue prints for making and maintaining an organism is kept in the form of DNA. A template of how to produce a particular protein can be generated from this DNA – that template is called RNA – and that is used to produce a protein (via a process called translation). A large part of that protein production process is conducted within the endoplasmic reticulum.
Once the GCase enzyme is correctly folded, it is transported (by chaperone proteins like LIMP-2) from the endoplasmic reticulum to small bags of digestive enzymes that are called lysosomes, which can be found floating around inside of cells.
 Source: NCBI
Source: NCBI
How do these lysosomes work?
On a relatively continual basis, small parts of a cell membrane are being brought inside the cell. This is a process called endocytosis.
It occurs when the cell needs to consume resources from the outside world in order to find what it requires to function and survive. As a section of cell membrane is brought into the cell, it forms a small spherical bag which is called a vesicle. Given the process by which these outer membrane vesicles are formed, they is referred to as endosomes (sometimes it is also called a vacuole).
 Source: Socratic
Source: Socratic
Once the endosome is inside the cell and detached from the rest of the membrane, it will bind to another vesicle – the lysosome. And as I mentioned above, lysosome is a small bag that is full of digestive enzymes, which help to break down the contents of the endosome.

How lysosomes work. Source: Prezi
The lysosome will fuse with the endosome/vacuole and the enzymes from the lysosome will mix with the material in the vacuole and digest it (or it break down into more manageable components).
This enzymatic process works in a very similar fashion to the commercial products that you use for washing your clothes.
 Enzymatic degradation. Source: Samvirke
Enzymatic degradation. Source: Samvirke
The reagents that you put into the washing machine with your clothes contain a multitude of enzymes, which help to break down the dirty, bacteria, flakes of skin, etc that cling to your clothes. Each enzyme breaks down a particular protein, fat or such like. And this is very similar to the collection of enzymes in the lysosome. All of them are needed to break down all of the contents of the endosome.
GCase is one of these enzymes in the lysosome.
Now, it has previously been assumed that if one of those enzymes – such as GCase – is faulty (due to a genetic mutation), then the enzymatic process will be disrupted and this could result in the build up of un-degraded material over time.
Ok, so GCase is an enzyme that helps to breakdown waste. But how is it associated with Parkinson’s?
Well, if one of the enzymes – such as GCase – in the waste disposal system is faulty (due to a genetic mutation), then the enzymatic process is disrupted, and this could result in the build up of un-degraded material over time. Such a build up is believed to put stress on cells and possibly even kill them.
And this could be what is happening in Parkinson’s.
|
# RECAP #1: Glucocerebrosidase (also known as GCase) is an enzyme that has long been known as being a key component of the waste disposal system in cells. Genetic variations in an area of DNA called the GBA1 gene are associated with an increased risk of developing Parkinson’s. # |
So there is a build up of the proteins that GCase breaks down in people with Parkinson’s?
Sooooo, this is where the data doesn’t line up nicely with the theory.
What do you mean?
I mean that glucocerebroside (the main target of GCase enzymatic activity) doesn’t build up in the brains of people with GBA-associated Parkinson’s (people with Parkinson’s and GBA1 genetic variants).
?????
This paper here was one of the first to report this finding:
 Title: No evidence for substrate accumulation in Parkinson brains with GBA mutations.
Title: No evidence for substrate accumulation in Parkinson brains with GBA mutations.
Authors: Gegg ME, Sweet L, Wang BH, Shihabuddin LS, Sardi SP, Schapira AH.
Journal: Mov Disord. 2015 Jul;30(8):1085-9.
PMID: 26096906 (This report is OPEN ACCESS if you would like to read it)
In the study, the researchers analysed samples of brain tissue from individuals with GBA-associated Parkinson’s as well as from cases of idiopathic Parkinson’s and compared the results with samples from age matched control brains. While the GBA-associated Parkinson’s cases exhibited 50% reductions in GCase activity, the levels of glucocerebroside were no different between the three groups.
I repeat: ?????
Yeah, I know. It has been a real mystery for the Parkinson’s research field as to why we don’t see a build up of undigested glucocerebroside over time.
One that is made more complicated by the fact that other proteins, such as Parkinson’s associated protein alpha synuclein do build up in the brains of people with GBA-associated Parkinson’s.
And we have previously discussed other research here on the SoPD that tries to explain this conundrum (Click here to read that post).
But it has also made people ask what else might GCase be doing that could help to explain this mystery and it’s association with Parkinson’s.
And this brings us back to the paper being reviewed in today’s post.
Ok, so the researchers wanted to know which proteins interact with GCase. How did they do that?
They used a technique called immunoprecipitation to do their study.
And before you ask, immunoprecipitation is a method by which researchers can label and isolate a protein of interest (like GCase) from cells to more closely analyse it. They can do this using ‘biological flags’ (in the form of antibodies) that attach themselves to and label the target protein. They can then separate the labelled protein from other cellular material (using various methods). Once isolated, the protein (and whatever is attached to it) can be carefully analysed.
This video explains immunoprecipitation better than I do:
Ok, so they isolated GCase from cells. Then what did they do?
Well, do you see in the paragraph above where I just wrote “Once isolated, the protein (and whatever is attached to it) can be analysed”? The important part of that sentence is the “whatever is attached to it”. The researchers analysed the different proteins that GCase was attached to and made a list of the main interactors.
And here is where they noticed something really interesting…
When they looked at the list of proteins that GCase is interacting with, they found many of the “usual suspects” (such as LIMP2, calnexin, calreticulin, and DNAJB11). All of these proteins are associated with lysosomal and cellular waste disposal.
But there was something else in the data that was rather intriguing and caught the attention of the researchers:
Almost 20% (or 1/5) of the top 100 binding partners of GCase were proteins associated with mitochondria.
What are mitochondria?
Mitochondria are tiny bean shaped objects that reside inside of almost every cell in your body. They function as the power stations of each cell and there are hundreds (often thousands) of them per cell, being moved around internally as needs dictate.
 Mitochondria. Source: Ohiostate
Mitochondria. Source: Ohiostate
It is important to know that mitochondria are essential for normal cellular function, AND that issues with mitochondrial function are associated with Parkinson’s. In fact, many of the genetic risk factors associated with Parkinson’s, are linked to proteins that are involved in normal mitochondrial function.
Thus, it is REALLY curious that GCase is interacting with proteins associated with mitochondria.
Interesting. What did they do next?
They repeated exactly the same experiment, but this time they used cells with genetic variations in their GBA1 gene (remember, this is the region of DNA that provides the instructions for making GCase). As a result of the genetic variations, these cells produced a mutant form of the GCase protein.
And when they looked at the data from this study, the researchers found that there was an increase in interactions with proteins involved in mitochondrial protein quality control (for example, HSP60 and LONP1). These are proteins that make sure the mitochondria are only receiving functional proteins.
Given that genetic variations in the GBA1 gene lead to GCase protein that is not folded properly, it isn’t really a big surprise that there is an increase in the mitochondrial protein quality control system. But this finding did further support the idea that GCase could be doing something in the mitochondria.
And so the investigators took a deeper look.
To firstly confirm that both normal and mutant versions of GCase protein can be imported into mitochondria, they conducted a series of localisation experiments. As part of this investigation, they labelled GCase protein and also labelled mitochondria with different coloured dyes, and found that they did overlap inside of cells, suggesting that the protein is being transported to the mitochondria.
In addition to the localisation experiments, the researchers also identified two previously unrecognised “mitochondrial targeting signal“-like regions on GCase protein. These are sections of the GCase protein that help cells to traffic the enzyme to the mitochondria. When they blocked these regions, GCase was no longer transported to the mitochondria of cells.
Ok, so GCase is transported to the mitochondria. But does it actually have any function or effect on them?
Inside the mitochondria, the researchers found that GCase promotes the maintenance and function of complex I in the mitochondrial electron transport chain.
You know what I’m going to ask, don’t you?
Yes. The mitochondrial electron transport chain is a series of protein structures that lie on the inner wall of mitochondria and produce adenosine triphosphate (or ATP).
 Source: Youtube
Source: Youtube
ATP is the energy-carrying molecule found in the cells of all living things. It is absolutely essential as it provides energy to drive many processes in living cells. An explanation of the entire electron transport chain requires a whole website of its own. This video, however, does an excellent good job of providing an overview:
What is important to understand here is that Complex I is the first of the five components of the mitochondrial electron transport chain, and it is involved with the production of energy (ATP) in cells..
And the idea that GCase is interacting with it is very interesting!
But the researchers found that mutant forms of the GCase protein (such as those associated with genetic variations in the GBA1 gene) impair Complex I stability and function.
What does that mean?
The investigators found that Complex I activity in the mitochondria was significantly reduced in cells carrying GBA1 genetic variations.
And when they looked at different types of neurons in cell culture, the researchers found that there was a higher level of dysregulation of mitochondrial energy metabolism in dopamine neurons in the cells with GBA1 genetic variations. than other types of neurons (when compared to control dopamine neurons).
|
# # RECAP #2: New research indicates that GCase is also being transported to the mitochondria inside of cells. Mitochondria are the power stations of cells and GCase interacts with proteins involved in the generation of energy in mitochondria. # # |
Interesting. Is this the first time anyone has ever reported this? GCase and mitochondria?
Yes, it is. But previous research has pointed towards mitochondrial differences in the context of GBA1 genetic variants.
For example, this report published in 2013:
 Title: Mitochondria and quality control defects in a mouse model of Gaucher disease–links to Parkinson’s disease.
Title: Mitochondria and quality control defects in a mouse model of Gaucher disease–links to Parkinson’s disease.
Authors: Osellame LD, Rahim AA, Hargreaves IP, Gegg ME, Richard-Londt A, Brandner S, Waddington SN, Schapira AHV, Duchen MR.
Journal: Cell Metab. 2013 Jun 4;17(6):941-953.
PMID: 23707074 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers investigated the effect of removing the GBA1 gene completely from mice. In addition to lysosomal issues, the investigators found that “mitochondria were dysfunctional and fragmented“, with reduced electron chain complex activities and energy production.
They concluded, however, that this mitochondrial outcome was probably associated with reduced waste disposal activity.
Other research groups have also reported mitocondrial issues in the absence of proper GCase activity (Click here to read a good review on this topic – see section 1.5 in particular).
OK. So what does it all mean?
Before we sum up, there is one other detail I wanted to raise regarding this topic.
And what is that?
There are several agents that are currently being clinically tested for their ability to elevate GCase levels. One of those is the cough medicine ambroxol (Click here to read a previous SOPD post about ambroxol).
 Source: Skinflint
Source: Skinflint
Full disclosure: The author of this blog is an employee of Cure Parkinson’s, which is an international funder of research focused on slowing/stopping/reversing Parkinson’s, and the charity has been supporting the clinical repurposing of ambroxol for PD (Click here to read more about this).
Understood. So why are you mentioning ambroxol here?
While it might be occurring via GCase independent-mechanisms, it is interesting to note that there have been reports that ambroxol improves mitochondrial function.
Reports such as this one here:
 Title: Effects of ambroxol on the autophagy-lysosome pathway and mitochondria in primary cortical neurons.
Title: Effects of ambroxol on the autophagy-lysosome pathway and mitochondria in primary cortical neurons.
Authors: Magalhaes J, Gegg ME, Migdalska-Richards A, Schapira AH.
Journal: Sci Rep. 2018 Jan 23;8(1):1385.
PMID: 29362387 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers used mouse neurons grown in cell culture to assess the effect of ambroxol on various aspects of cellular biology. They found that levels of mitochondria content increased with ambroxol treatment, and this was associated with elevated levels of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α).
Other research reports have indicated improvements in mitochondrial function following ambroxol treatment (Click here to read another example).
As I say, this effect of ambroxol could be independent of any GCase involvement, but it would be interesting to investigate if ambroxol (or any of the other GCase activators) is elevating levels of GCase in the mitochondria (and enhancing function there as a result).
So what does it all mean?
We started this post with the curious case of Adam Rainer, who changed from a dwarf into a giant during his lifetime. I now wonder if that particular story was such a good way to start this post. While our attention in this post has been focused on new research suggesting a novel function for the Parkinson’s-associated protein GCase, the data does not suggest a change for this protein that is as dramatic as the situation was for poor old Adam. Maybe I need to give more thought to my introductions.
We are regularly confronted in science with new perspectives or new functions for particular proteins. And I love the breaking of dogma (“GCase is just a lysosomal enzyme”). But challenging dogma is no easy feat. The current study will require independent replication and further investigation to determine if the results are physiologically relevant. Maybe GCase is just a lysosomal enzyme, and occasionally it finds itself in the mitochondria.
It would be very interesting from the stand point of Parkinson’s if this new function of GCase is ‘a thing’ (as my daughter likes to say). For a long time, dysfunction in the mitochondria has been ‘a thing’ associated with Parkinson’s and suspected to play a key role in the development and progression of the condition. By interacting with mitochondrial proteins, the PD-related protein GCase could be directly associating itself with the ground-zero of Parkinson’s. I look forward to following this research further, particularly if some of the GCase activator agents (like ambroxol) being developed for PD are found to increase transportation of GCase to the mitochondria and improve their function.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from

