|
# # # # The diagnostic process for Parkinson’s has been problematic for a long time. Individuals presenting the symptoms often need several clinical evaluations, and confirmation using a brain imaging technique. A biological test for the condition has been lacking and would help tremendously. Recently, however, research (supported by the Michael J Fox Foundation for Parkinson’s research) has indicated that this could be about to change. In today’s post, we will explore recently published research highlighting a new potential biomarker test for Parkinson’s. # # # # |
On the 27th June, 1997, a research report was published in the prestigious scientific journal ‘Science’ that would change the world of Parkinson’s forever.
And I am not exaggerating or overstating here. I know I can sometimes be a little over the top, but the research report in question very much changed the world of Parkinson’s research.
The discovery that tiny variations in a region of DNA that scientists refer to as “alpha synuclein” could increase the risk of developing Parkinson’s gave researchers their first real insights into some of the biology that could potentially be underlying the condition (Click here to read a previous SoPD post on this discovery):

Title: Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease.
Authors: Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL.
Journal: Science. 1997 Jun 27;276(5321):2045-7.
PMID: 9197268
And then – remarkably just two months later – the results of another study were published in the journal ‘Nature’, and these would further cemented alpha synuclein’s place in Parkinson’s research.
In this second research paper, the investigators showed that alpha synuclein was present in “Lewy bodies” – densely packed spheres of protein inside of cells that are one of the characteristic features of the Parkinsonian brain:
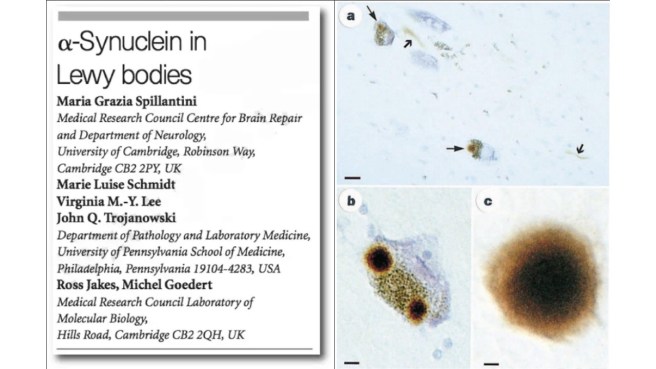 Title: Alpha-synuclein in Lewy bodies.
Title: Alpha-synuclein in Lewy bodies.
Authors: Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M.
Journal: Nature. 1997 Aug 28;388(6645):839-40.
PMID: 9278044
And very suddenly, this poor little protein became public ‘enemy number one’ for the Parkinson’s research community and everyone started digging into the biology associated with it with the hope of finding new avenues for therapeutic intervention and biomarkers for Parkinson’s.
What exactly is alpha synuclein?