|
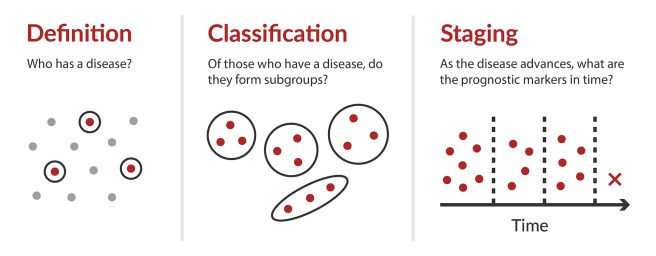
# # # # The diagnosis of Parkinson’s has always been a decision based on clinical observations. It involves the presentation of a triad of motor symptoms (slowness of movement, rigidity, and a resting tremor) as well as additional non-motor features. There have been efforts to develop staging systems, based on these clinical symptoms (such as the Hoehn and Yahr scale and the Unified Parkinson’s Disease Rating Scale – UPDRS) which have helped with assessing the progression of the disease. But very recently researchers have been exploring the possibility of staging systems for Parkinson’s based on biological, rather than clinical features. In today’s post, we will examine two proposals for staging systems and we will consider what this means for the condition we currently know of as “Parkinson’s”. # # # # |
 Source: Sage
Source: Sage
In the 1940/50s, a French surgeon in Villejuif, France revolutionised the treatment of breast cancer.
Prof. Pierre Denoix developed a simple anatomic staging system which was dependent on the size of the tumor, nodal status, and metastatic findings. This simple, anatomically based approach became known as the TNM (tumor, node, metastases) staging system (Click here to read more about this).
Since that time, staging systems for other cancers have been developed and represent an important component of the diagnostic and treatment process. More sophisticated additional assessments have since been added, which have allowed for more targeted and personalised therapies to be employed. And this has led to significantly improved survival outcomes and better quality of life for cancer patients.
 Source: Sciencedirect
Source: Sciencedirect
Is there a staging system for Parkinson’s?
There are clinical staging systems, such as the Hoehn and Yahr scale, which was first proposed by Margaret Hoehn and Melvin Yahr in 1967.
 Title: Parkinsonism: onset, progression and mortality.
Title: Parkinsonism: onset, progression and mortality.
Authors: Hoehn MM, Yahr MD.
Journal: Neurology. 1967 May;17(5):427-42.
PMID: 6067254
Their original scale had 5 stages:
- Unilateral involvement, usually with minimal or no functional disability
- Bilateral or midline involvement without impairment of balance
- Bilateral disease: mild to moderate disability with impaired postural reflexes; physically independent
- Severely disabling disease; still able to walk or stand unassisted
- Confinement to bed or wheelchair unless aided
There have been some minor modifications to the original Hoehn and Yahr scale over the years, but it has largely remained the same.
But the problem with the Hoehn and Yahr scale (and other clinical rating scales like the Unified Parkinson’s Disease Rating Scale (or UPDRS)) is that it is a subjective judgement by the clinician. And how one clinician defines “mild to moderate disability” may differ significantly from another clinician’s opinion.
 Profs Melvin Yahr and Margaret “Peggy” Hoehn. Source: Parkinsonsecrets
Profs Melvin Yahr and Margaret “Peggy” Hoehn. Source: Parkinsonsecrets
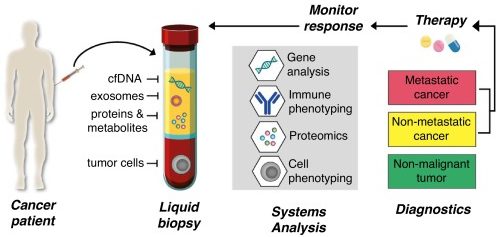
The difference between Parkinson’s and cancer is rather stark. What oncology has is a biologically defined, specific criteria.
In addition, when a person is diagnosed with a cancer, a biopsy will be taken and the clinician will be able to gauge exact what type of cancer they are dealing with. Specific genetic variations in the tumor will be identified and the patient will then be able to be treated with a particular cocktail of therapies that target their specific type of cancer.
This is the way disease should be dealt with – in a very personalised, targeted fashion.
The Hoehn and Yahr scale? The UPDRS?
Not so personalised.
So how can we improve this situation?
Well, researchers have been working hard on new biological ‘staging’ approaches for Parkinson’s, and proposals have recently been written and published.
What do they propose?
In April 2023, there was a meeting of experts to discuss the better classification and staging of Parkinson’s. This was the report of that meeting:
 Title: A Statement of the MDS on Biological Definition, Staging, and Classification of Parkinson’s Disease.
Title: A Statement of the MDS on Biological Definition, Staging, and Classification of Parkinson’s Disease.
Authors: Cardoso F, Goetz CG, Mestre TA, Sampaio C, Adler CH, Berg D, Bloem BR, Burn DJ, Fitts MS, Gasser T, Klein C, de Tijssen MAJ, Lang AE, Lim SY, Litvan I, Meissner WG, Mollenhauer B, Okubadejo N, Okun MS, Postuma RB, Svenningsson P, Tan LCS, Tsunemi T, Wahlstrom-Helgren S, Gershanik OS, Fung VSC, Trenkwalder C.
Journal: Mov Disord. 2024 Feb;39(2):259-266.
PMID: 38093469
This article is not available to the public. It is behind a paywall – yeah, I don’t understand either – but the Movement Disorder Society does offer a summary document here. In that summary, the authors highlight the “foundational principles for the development of a biological definition, classification, and staging system for Parkinson’s”. And those effort have led to the recent publication of two proposals for staging systems.
The first (in no particular order) is the SynNeurGe research diagnostic criteria:
 Title: A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria.
Title: A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria.
Authors: Höglinger GU, Adler CH, Berg D, Klein C, Outeiro TF, Poewe W, Postuma R, Stoessl AJ, Lang AE.
Journal: Lancet Neurol. 2024 Feb;23(2):191-204.
PMID: 38267191
This article is also behind a paywall, but some of the authors have offered an OPEN ACCESS review of the SynNeurGe system (Click here to read that review). In their proposal, the authors outline a three-component system (“SynNeurGe”), which depends on:
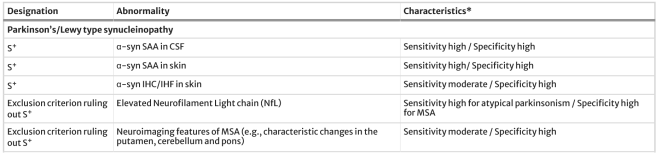
- The presence or absence of pathological α-synuclein (Syn) in a biopsied tissue or samples of cerebrospinal fluid (the liquid surrounding the brain). We have previously discussed α-synuclein and new assays exploring it as a biomarker for Parkinson’s (Click here to read that SoPD post):
 Source: Springer
Source: Springer
- Evidence of underlying neurodegeneration (Neur) defined by neuroimaging procedures (such as DAT-SPECT or Metabolic FDG PET brain imaging):
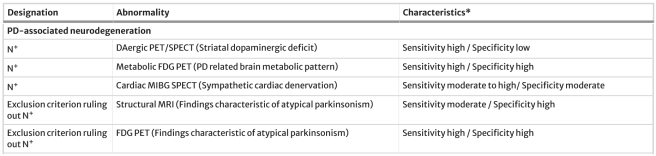 Source: Springer
Source: Springer
- Documentation of pathogenic gene variants (Ge) that cause or infer vulnerability to Parkinson’s:
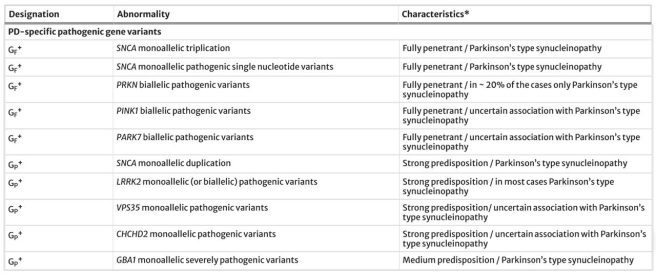 Source: Springer
Source: Springer
Rather than being a strict staging or classification system, the authors hope that SynNeurGe “offers a promising avenue for refining Parkinson’s diagnosis and understanding its underlying biology“.
In addition, they note that an individual who is Syn-negative (that is to say, they have no positive for pathological α-synuclein in their cerebrospinal fluid) may still qualify for a diagnosis of Parkinson’s in the case where they harbor a Parkinson’s genetic variant which may predispose vulnerability for Parkinson’s, but without associated synucleinopathy (we see this in some cases of LRRK2-associated Parkinson’s).
And they emphasize that “further research and validation are needed to ensure reliability and accuracy in clinical practice“.
The second staging proposal is the Neuronal α-Synuclein Disease Integrated Staging System (or NSD-ISS), which was outlined in this position paper:
 Title: A biological definition of neuronal α-synuclein disease: towards an integrated staging system for research.
Title: A biological definition of neuronal α-synuclein disease: towards an integrated staging system for research.
Authors: Simuni T, Chahine LM, Poston K, Brumm M, Buracchio T, Campbell M, Chowdhury S, Coffey C, Concha-Marambio L, Dam T, DiBiaso P, Foroud T, Frasier M, Gochanour C, Jennings D, Kieburtz K, Kopil CM, Merchant K, Mollenhauer B, Montine T, Nudelman K, Pagano G, Seibyl J, Sherer T, Singleton A, Stephenson D, Stern M, Soto C, Tanner CM, Tolosa E, Weintraub D, Xiao Y, Siderowf A, Dunn B, Marek K.
Journal: Lancet Neurol. 2024 Feb;23(2):178-190.
PMID: 38267190
And guess what? This report is not OPEN ACCESS either.
Bit of a theme going here.
The authors of this position paper suggested that it is time to redefine Parkinson’s and dementia with Lewy bodies as “Neuronal α-synuclein disease” rather than as individual clinical conditions. And they proposed a a new research biological framework for Neuronal alpha-Synuclein Disease and an integrated staging system (NSD-ISS).
This biological definition is based on three key ideas:
- Disease needs to be defined biologically using validated biomarkers
- Disease can be diagnosed in absence of clinical manifestations
- The same biology may result in different phenotypic presentations
Using this approach, symptoms are the result of the disease process, but do not define it.
The current NSD-ISS proposal involves seven distinct stages:
- Stage 0 which is the presence of fully penetrant pathogenic variants in SNCA gene
- Stage 1 which is divided into Stage 1A (the presence of neuronal aggregated a-synuclein protein alone) or Stage 1B (the presence of neuronal aggregated a-synuclein protein in combination with dopaminergic dysfunction). Crucially, this stage is asymptomatic – that is, the individuals will not exhibiting any clinical symptoms
- Stage 2 which is the presence of neuronal aggregated a-synuclein protein alone (Stage 2A) or in combination with dopaminergic dysfunction (Stage 2B). In both cases, there will be subtle clinical signs/symptoms, but they will be present without functional impairment
- Stages 3–6 which involve the presence of both neuronal aggregated a-synuclein protein and dopaminergic dysfunction. The clinical signs/symptoms presented will be present with progressively increasing severity of functional impairment.
Using such a system, Neuronal α-synuclein disease can be defined by the presence of pathological α-synuclein species detected in biological samples (such as cerebrospinal fluid) regardless of the presence of any specific clinical symptoms. They propose that individuals with pathological neuronal-α-synuclein aggregates are at risk of dopaminergic neuronal dysfunction, and this staging system (rooted in biological anchors) provides an opportunity for better and earlier intervention than the current clinical staging systems (eg. UPDRS).
One of the goals of any new biological staging effort should be to be able to begin treating people earlier, before any symptoms appear. If we can pick up pathological neuronal-α-synuclein aggregates in a biopsy (cerebrospinal fluid test) several years before symptoms begin to show themselves, then interventions can be made (be it pharmacological or lifestyle) to slow the onset:
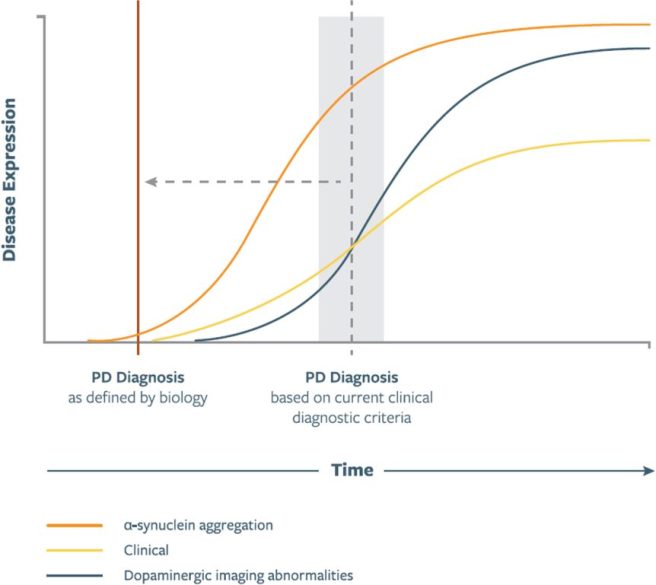 Source: JPD
Source: JPD
The NSD-ISS team do acknowledge that their criteria is restricted to sporadic “Parkinson’s” patients who have positive cerebrospinal fluid aggregated a-synuclein protein, and in terms of individuals carrying a genetic risk factor they only include rare SNCA genetic cases which are considered more causal. Thus, individuals with LRRK2-associated Parkinson’s (for example) and no neuronal-α-synuclein aggregates will fall outside of this system.
Interesting. How solid are these staging system proposals?
Well, it has to be said that these are a good first attempts at staging biological systems, and they provide useful models that can now be tested and developed. Based on our current understanding of things, it feels like a step in the right direction. And given how long neurology has relied solely on clinical observation (due to the inaccessibility of the brain), a step in any direction will hopefully provide new insights.
Obviously, both proposals rely heavily on the alpha synuclein angle, and don’t (yet) take into account other biomarkers that are being developed (such as DOPA decarboxylase levels in cerebrospinal fluid (Click here to read an SoPD post on this topic) or mitochondrial DNA damage (Click here to read an SoPD post on this topic)). The reliance on alpha synuclein begs the question: By removing aggregated alpha synuclein, will we consider an individual “cured” (or in remission) of Stage 1A or Stage 2A NSD?
Is it really going to be that easy?
As a Parkinson’s advocate recently said to me when we were discussing some of this recent research, “if our understanding of the biology is wrong, then we are building on a foundation of sand“. But science is not about going from fact to fact, rather we move forward via trial n’ error and a lot of hypothesis testing.
So what does it all mean?
This post reminds me of a quote from the revered neurologist Jean-Martin Charcot:
“Disease is very old and nothing about it has changed. It is we who change, as we learn what was formerly imperceptible” (from De L’Expectation en Médecine)
As you may have picked up throughout the post, I am frustrated that all of the primary papers discussed are behind paywalls. As someone without access to the literature, I am left shaking my head as to what thought process or strategy decided this. While most disease fields have embraced the contribution that the patient voice can make and provide access to articles (even via preprint repositories such as medRxiv or bioRxiv), neurology is still too conservative. It seemingly needs to be dragged kicking and screaming to any new concepts and paradigms. The patient perspective would be useful (one might say necessary) in this domain. There will be much more to come in this biological staging space, and it would be wonderful to get a patient perspective on it to add to the mix.
If readers are interested in this topic of biological staging, I can recommend reading Prof Michael Okun’s wonderful “Parkinson Secrets” post on this topic (Click here to read that). As more biomarker work comes forward and gets independently validated across large cohorts, we will hopefully see an evolution in these staging proposals. They may need to be completely re-arranged if future data dictates, but such is the nature of scientific research. As I suggested above, the motivation to explore new diagnostic/staging criteria should be encouraged, as all of this effort will all bring new insight and clarity. We must not fall back on to “the way it has always been done” mentality as this is not how change/progress occurs.
# # # # # # # #
ADDENDUM – October 2024:
There has been an interesting response to these proposals for staging systems for “Parkinson’s”. There have been a lot of responses to the original papers, and thankfully many of them are in the public domain (Click here, here, here, here, here, and here to read some of the correspondences), while others remain behind paywalls (Click here and here for some examples).
One of the best viewpoints I have seen has come from the neuropathologists:
 Title: Biomarker-Based Approach to α-Synucleinopathies: Lessons from Neuropathology.
Title: Biomarker-Based Approach to α-Synucleinopathies: Lessons from Neuropathology.
Authors: Kovacs GG, Grinberg LT, Halliday G, Alafuzoff I, Dugger BN, Murayama S, Forrest SL, Martinez-Valbuena I, Tanaka H, Kon T, Yoshida K, Jaunmuktane Z, Spina S, Nelson PT, Gentleman S, Alegre-Abarrategui J, Serrano GE, Paes VR, Takao M, Wakabayashi K, Uchihara T, Yoshida M, Saito Y, Kofler J, Rodriguez RD, Gelpi E, Attems J, Crary JF, Seeley WW, Duda JE, Keene CD, Woulfe J, Munoz D, Smith C, Lee EB, Neumann M, White CL 3rd, McKee AC, Thal DR, Jellinger K, Ghetti B, Mackenzie IRA, Dickson DW, Beach TG.
Journal: Mov Disord. 2024 Oct 3. Online ahead of print.
PMID: 39360851 (This report is OPEN ACCESS if you would like to read it)
In this OPEN ACCESS viewpoint, the researchers welcome the “initial attempts to redefine these diseases incorporating biological constructs, particularly for the early and in vivo diagnosis of these disorders“. But they express “caution” when considering “mere detection of seeding of misfolded α-synuclein in bodily fluids or peripheral tissue as α-synucleinopathy reflecting a brain disease“. They rightly point out that “currently α-synuclein seeding amplification assays (SAA) lack sensitivity and specificity for brain region and cell type, features known from neuropathology to be critically important to clinical outcomes”.
The authors also note the inconsistent presence of alpha synuclein pathology in other conditions (such as Alzheimer’s), and “lumping clinicopathological entities based on the presence of α-synuclein seeding alone would not decrease heterogeneity“. They suggest that “combining Alzheimer’s biomarkers for better stratification” may be needed to more clearly determine subtypes and stages.
The authors conclude on a wonderfully positive and collaborative note by saying “There is much research to be done to ensure their [biomarkers] appropriate & effective use in clinical settings. The neuropathology community is eager to work with our clinical & neuroimaging colleagues to achieve this important goal“.
One interesting use of the staging system has already been applied to previous clinical trials cohorts, to evaluate “the baseline heterogeneity of individuals currently defined as early Parkinson’s“. This is the report in question:
 Title: Neuronal alpha-Synuclein Disease integrated staging system performance in PPMI, PASADENA, and SPARK baseline cohorts.
Title: Neuronal alpha-Synuclein Disease integrated staging system performance in PPMI, PASADENA, and SPARK baseline cohorts.
Authors: Dam T, Pagano G, Brumm MC, Gochanour C, Poston KL, Weintraub D, Chahine LM, Coffey C, Tanner CM, Kopil CM, Xiao Y, Chowdhury S, Concha-Marambio L, DiBiaso P, Foroud T, Frasier M, Jennings D, Kieburtz K, Merchant K, Mollenhauer B, Montine TJ, Nudelman K, Seibyl J, Sherer T, Singleton A, Stephenson D, Stern M, Soto C, Tolosa E, Siderowf A, Dunn B, Simuni T, Marek K; Parkinson’s Progression Markers Initiative.
Journal: NPJ Parkinsons Dis. 2024 Sep 27;10(1):178.
PMID: 39333167 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers pooled baseline data from recent clinical trial cohorts and the Michael J Fox Foundation’s Parkinson’s Progression Markers Initiative.
Across the three studies, there were 1741 participants who had alpha synuclein seeding amplification assay data (each individual sample was analyzed in triplicate). Of these 1030 (or 59%) had positive results consistent with NSD. When they looked at just the PPMI data (1,553 evaluable cases), they saw an interesting breakdown of the cohort that could be interesting to follow up (only 64% of LRRK2-associated Parkinson’s cases and 33% of PARKIN-associated Parkinson’s cases were NSD+):
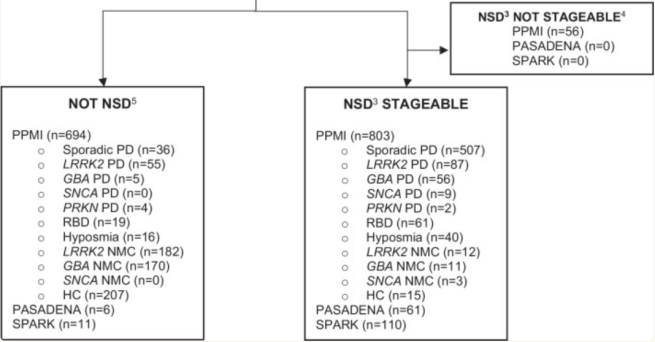 ‘Not stageable’ = no DAT-scan data. Source: PMC
‘Not stageable’ = no DAT-scan data. Source: PMC
But the surprising detail in this study was the heterogeneity of the “early Parkinson’s” cohorts. In the PPMI study, most of the cases with clinically diagnosed Parkinson’s met the criteria for stage 3 (65% sporadic Parkinson’s, 61% LRRK2-associated Parkinson’s, and 59% GBA-associated Parkinson’s) and this was at their baseline data collection. Equally, in the PASADENA and SPARK clinical trials, 66% and 55% of participants were stage 3, respectively (26% and 25% were Stage 2B, respectively). These differences are important – particularly in a clinical trial, where participants are being compared in the testing of experimental agents – as the researchers found that the rate of progression varied between stages.
Another interesting development has been the use of the NSD-ISS system in the Michael J Fox Foundation’s Pathway to Prevention (P2P) study. The P2P study is an exciting new endeavour, which is seeking to test new experimental therapies in people before they are diagnosed with “Parkinson’s”. The trial team will be recruiting participants from the PPMI study who fall into the definition of NSD Stage 2B (neuronal aggregated a-synuclein protein in combination with dopaminergic dysfunction – click here to read the protocol – a PDF file). It will be interesting to see if this carefully defined biological cohort provides better outcomes than the previous clinical trial recruitment criteria. It is certainly worth trying! For those interested in learning more about designing clinical trials for testing interventions in people before they are diagnosed with Parkinson’s, click here to read an interesting review.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from MovementDisorders