|
# # # # People diagnosed with REM sleep behavior disorder (or RBD) have a higher risk of developing Parkinson’s. RBD is a sleep condition in which the affected individual physically acts out their dreams as they sleep. Usually when we are dreaming, our bodies become momentarily paralysed. But sufferers of RBD loss this ability and begin moving about in bed, reacting to their dreams. Recently, researchers have been testing a treatment for vertigo (called Tanganil) in people with RBD, and the results of a small pilot study are rather remarkable. In today’s post, we will look at what RBD is, what the new pilot study found, and what this could mean for Parkinson’s. # # # # |
 Dreaming. Source: Psypost
Dreaming. Source: Psypost
When we sleep, our brain (and body) pass through different phases of slumber. In general, there are two broad segments of sleep:
- Rapid eye movement (REM) sleep, and
- Non-rapid eye movement (NREM) sleep.
And we pass through these phases in a wave-like cycles across the night:
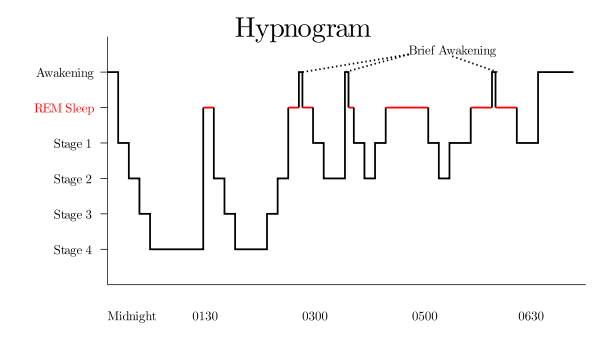 Stages of sleep. Source: Wikipedia
Stages of sleep. Source: Wikipedia
In addition, there are different stages that make up the NREM sleep parts of these cycles (stage 1-4, on the left hand side of the image above), which we pass through on our way down to stage 4 and back again.
These stages exhibit different patterns of brain activity, which – when recorded – look like this:
 Brain activity during stages of sleep. Source: Homesteadschools
Brain activity during stages of sleep. Source: Homesteadschools
The brain is most active during REM sleep, and this is the period during which we dream. The first period of REM sleep begins about 90 minutes after you fall asleep. It typically lasts for approximately 10 minutes. Each period of REM sleep for the rest of the night gets longer and longer.
When we dream, there are cells in the brainstem region of the central nervous system that inhibit our ability to move. So while we may be dreaming of being a Hollywood action movie star at the Oscars, or perhaps walking on the moon, or maybe turning up at your high school reunion naked, our bodies are momentarily paralysed. One assumes that this mechanism has evolved within our species over time for our own safety – to prevent us from hurting ourselves or others. It is interesting to note that the phenomenon of sleepwalking occurs during the deeper states of NREM sleep when we are not temporarily paralysed (source).
This is very interesting, but what does it have to do with Parkinson’s?
Well, REM sleep behaviour disorder (RBD) is a chronic sleep condition characterized by people physically acting out their dreams (unknowingly) while they are asleep. It affects about 1% of the general US population, affecting 2% of people over the age of 50.
 Acting out your dreams. Source: Sleepreviewmag
Acting out your dreams. Source: Sleepreviewmag
Postmortem analyses of people who have passed away with RBD have noted that the cells in the brain stem responsible for inducing the temporary paralysis are lost in RBD. Thus, there is less of a handbrake to stop people from responding to the contents of their dreams. RBD can be extremely distressing for sufferers and (more importantly) for partners who may be sharing a bed with them.
In 1996, it was noted by several research groups that individuals with RBD exhibited a higher risk of developing Parkinson’s. In February of that year, researchers at the University of Minnesota Medical School provided a longitudinal report on a group of 29 men (50 years of age or older) who had been diagnosed with RBD. They noted that 38% (11/29) were eventually diagnosed with a parkinsonian disorder (Parkinson’s, Multiple Systems Atrophy, etc – click here to read more about this report).
One month later, this report from Prof Stanley Fahn and colleagues was published:
 Title: Rapid eye movement sleep behavior disorder preceding Parkinson’s disease with therapeutic response to levodopa.
Title: Rapid eye movement sleep behavior disorder preceding Parkinson’s disease with therapeutic response to levodopa.
Authors: Tan A, Salgado M, Fahn S.
Journal: Mov Disord. 1996 Mar;11(2):214-6.
PMID: 8684394
In this study, the investigators presented three case studies of patients whose RBD preceded the onset of Parkinson’s by several years. They also noted that both the symptoms of Parkinson’s and RBD improved with levodopa treatment.
Since then there have been many more studies reporting RBD as a ‘prodromal’ marker of Parkinson’s (and other Parkinsonisms such as dementia with Lewy body, and multiple system atrophy). It is believed that 70% of individuals with RBD will develop one of these conditions within 12 years of their diagnosis (Click here to read more about this). As such, the diagnosis of RBD is being viewed by many researchers are a good time point to initiate interventions to prevent the future development of other issues.
|
# RECAP #1: RBD is a sleep condition in which sufferers physically (but unconsciously) react to their dreams in bed. People with RBD have a higher risk factor of developing Parkinson’s. # |
So how are researchers trying to intervene?
Yes, the hope is that we can treat people with RBD and this will stop them from going on to develop Parkinson’s or something else.
There have been some really interesting reports on this idea of intervening, and careful consideration is being given to the designing of clinical trials to prevent Parkinson’s (Click here to read more about this).
And researchers have been developing agents to test in people with RBD to see if they can firstly improve the symptoms of RBD, but also slow down the processes leading on to Parkinson’s.
Recently a report was published which provides an interesting example:
 Title: Acetyl-DL-leucine in two individuals with REM sleep behavior disorder improves symptoms, reverses loss of striatal dopamine-transporter binding and stabilizes pathological metabolic brain pattern-case reports.
Title: Acetyl-DL-leucine in two individuals with REM sleep behavior disorder improves symptoms, reverses loss of striatal dopamine-transporter binding and stabilizes pathological metabolic brain pattern-case reports.
Authors: Oertel WH, Janzen A, Henrich MT, Geibl FF, Sittig E, Meles SK, Carli G, Leenders K, Booij J, Surmeier DJ, Timmermann L, Strupp M.
Journal: Nat Commun. 2024 Sep 2;15(1):7619.
PMID: 39223119 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers tested a vertigo treatment called Tanganil (also known as acetyl-DL-leucine) on two individuals that they had been assessing for RBD.
What is Tanganil/acetyl-DL-leucine?
Acetyl-DL-leucine is a medications that has been used over-the-counter to treat vertigo in France for over 65 years, sold under the brand name Tanganil. More recently it has been used in the treatment of cerebellar ataxia (a condition that causes a loss of muscle coordination, resulting in difficulty with balance, walking, speech, swallowing, and eye movements).
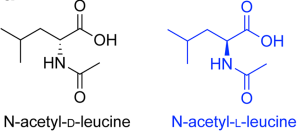
Acetyl-DL-leucine is one form of a modified leucine amino acid (Acetyl-leucine), of which there are two forms:
- Acetyl-DL-leucine/Tanganil (obviously)
- N-acetyl-L-leucine (levacetylleucine – more on this one later)
Acetyl-DL-leucine is a 1:1 mixture of enantiomers of acetyl-leucine.
What are enantiomers?
Enantiomers are a pair of molecules that exist in two forms that are mirror images of one another but cannot be superimposed one upon the other. Enantiomers are in every other respect chemically identical.
The enantiomers of acetyl-leucine are the inactive D-form and the bioactive enantiomer, the L-form of acetyl-leucine.
How does acetyl-DL-leucine work in the treatment of vertigo?
The mechanisms of action of acetyl-DL-leucine for vertigo are not fully understood.
Electrophysiological studies have shown that administration of acetyl-DL-leucine stabilises membrane potential of hyper- and hypopolarized neurons, meaning that acetyl-DL-leucine can help normalise neuronal function (Click here to read more about this).
Ok, but why were the researchers treating the two RBD patients with acetyl-DL-leucine?
Because in 2020, the research team involved with this report treated a Parkinson’s patient (who also suffered from Restless Legs Syndrome and RBD) with acetyl-DL-leucine.
What is Restless Legs Syndrome?
Restless Legs Syndrome is a neurological disorder that causes individuals to experience an uncomfortable feeling in the legs, along with an overwhelming urge to move them.
Given its ability to normalise neuronal function, the researchers were hoping that acetyl-DL-leucine treatment would help to normalise neuronal activity in Restless Legs Syndrome.
Ok, got it. So the researchers treated a person with restless legs syndrome and RBD with acetyl-DL-leucine. What did they find?
Well, they reported that within 2 weeks of initiating acetyl-DL-leucine treatment, the patient’s restless legs syndrome symptoms had substantially reduced.
Interesting.
Yeah, but more interesting: After 5 weeks of acetyl-DL-leucine treatment, the patient reported a reduction in the number of aggressive dreams they were experiencing and a reduction in their episodes of acting out their dreams.
The investigators reported these results in this paper:
 Title: Acetyl-DL-leucine improves restless legs syndrome: a case report
Title: Acetyl-DL-leucine improves restless legs syndrome: a case report
Authors: Fields, T., Schoser, B., Oertel, W. & Strupp, M.
Journal: J. Neurol.(2021) 268, 2595–2596.
PMID: 34052888
Fully aware that the initial finding was an N-of-1 study (just one participant), the researchers next recruited two additional RDB patients and asked them if they would be prepared to be treated chronically with acetyl-DL-leucine. Both agreed and underwent some baseline measures that could be used for comparison as the study progressed. Both of the RBD patients had brain imaging. Specifically, they had dopamine-transporter single-photon emission computerized tomography (DAT-SPECT).
What is DAT-SPECT?
Dopamine-transporter single-photon emission computerized tomography (DAT-SPECT) involves the injection of a radioactive ligand into the blood. This ligand (called DATscan) specifically binds to a protein called – you guessed it – dopamine transport (or DAT) .
And let me guess: Dopamine transporter protein is involved with transporting dopamine?
You are a genius.
DAT sits on the outside membrane of dopamine neurons and helps to recycle free floating dopamine back into the cell to be degraded or used again.
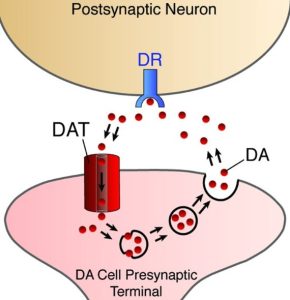 Dopamine Transporter. Source: Nature
Dopamine Transporter. Source: Nature
By having a ligand that can attach to dopamine transporter protein, researchers have a very useful tool for labelling dopamine neurons in the brain.
Why is this useful?
Because as people with Parkinson’s lose their dopamine neurons – a fundamental pathological characteristic of Parkinson’s – this loss can be measured using DAT-SPECT. And in people with RBD, who may be just starting to lose their dopamine neurons, DAT-SPECT can be used to assess the rate of loss and be used to determine if an experimental intervention is having any effect on slowing the progression of RBD to other neurodegenerative conditions, such as Parkinson’s.
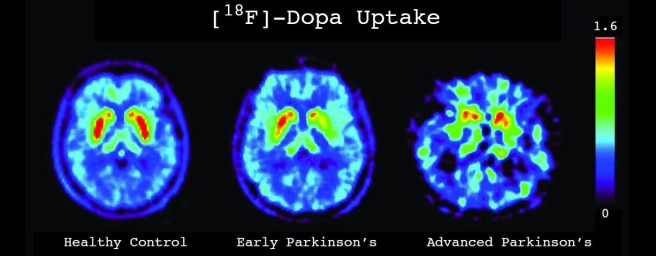 Source: Davisphinneyfoundation
Source: Davisphinneyfoundation
In the image above you can see a birds-eye view (looking down from above) of DAT-SPECT images of three brains – a normal control (left), a recently diagnosed Parkinson’s case (middle), and a more advanced case (right). On either side of the brains there are red spots, which designate the putamen – the site in the brain where most of the dopamine is released and where most of the dopamine transporter protein in located. Note that as Parkinson’s progresses (from early Parkinson’s to more advanced Parkinson’s), there is less red in this region of the brain.
Ok, so what were the baseline measures like for the two RBD patients who received acetyl-DL-leucine?
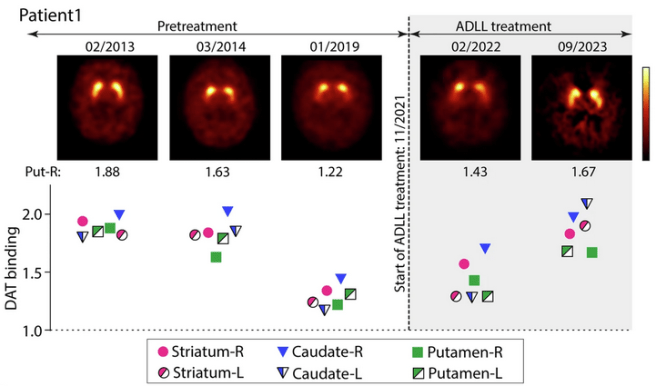
Patient 1 was a female who reported starting to experience the symptoms of RBD in 2006, and received a diagnosis of RBD in 2011. She underwent four pretreatment DAT-SPECT scans (in Feb 2013, March 2014, April 2016, and January 2019). In November 2021, this patient began taking acetyl-DL-leucine. Three months later, she received another DAT-SPECT scan (February 2022) and then she had another one in September 2023 after 22 months of continuous acetyl-DL-leucine therapy.
At the start of treatment, patient 1 had a “RBD-severity sum score” (RBD-SS-3) of 21, and an anosmia score (using the Threshold-Discrimination-Identification (TDI) test) of 6 (the score range of the TDI is 1 and 48, with lower scores indicating limited sense of smell). The RBD-SS-3 score is a scale that measures the severity of RBD symptoms, and scores range from 0–84, with higher scores indicating greater severity. Three weeks after starting acetyl-DL-leucine treatment, the patient’s RBD-SS-3 score had decreased to just 5 and she reported that her aggressive dreams had stopped almost completely. And this effect was sustained for the entire 22 months of continuous acetyl-DL-leucine treatment.
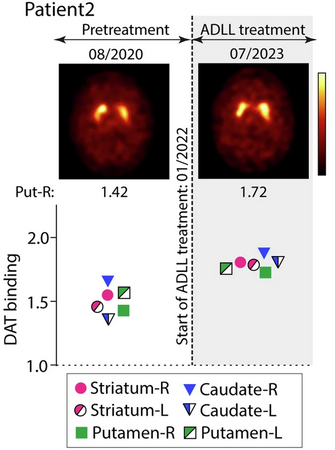
Patient 2 was a male who reported RBD symptoms starting in 2018. He was diagnosed with RBD in June 2020, and received his first DAT-SPECT scan in August 2020. This patient began acetyl-DL-leucine therapy in January 2022. After 12 months of continuous acetyl-DL-leucine treatment, he underwent another DAT-SPECT imaging session (January 2023) and then a second scan six months later (June 2023).
Patient 2 had a RBD-SS-3 score of only 7 before treatment was started, and the anosmia TDI-sum-score was 10 at baseline. Therefore, he was less severe in terms of their RBD symptoms than patient 1. Like patient 1, however, three weeks after starting acetyl-DL-leucine treatment, the RBD-SS-3 score reduced from 7 to 0, and the aggressive content of his dreams disappeared entirely. And again, these effects were maintained for 18 months of continuous acetyl-DL-leucine treatment.
Interesting. So acetyl-DL-leucine somehow ‘normalised neuronal activity’ associated with RBD?
Perhaps. The mechanism of action here is not clear, but acetyl-DL-leucine seems to have had an interesting effect on RDB symptoms.
And what about the DAT-SPECT brain imaging assessments?
Ah, so this is where the story gets really intriguing.
How so?
Below is an image presenting the timeline of assessments for Patient #1, both pretreatment and after initiation of acetyl-DL-leucine treatment. There are also dated representative DAT-SPECT images across the time line, with the putamens in each image presented in yellow. And below that is a graph showing the intensity scores from measuring DAT-binding across different regions of the putamen in those DAT-SPECT images. As you can see on the left hand side of the image, the DAT-SPECT image taken on the 02/2013 had a relatively normal appearance and the intensity scores below that image are all around 1.8-2.0.
 Source: PMC
Source: PMC
As you make your way from that first image to the next two (dated 03/2014 and 01/2019) you see a trend towards loss of DAT intensity, with the DAT intensity scores falling to below 1.5 across all of the putamen regions in the 01/2019 assessment. Then the researchers initiated acetyl-DL-leucine treatment (in 11/2021) and the DAT intensity scores began to rise at the next assessment (dated 02/2022) and appear to have returned to the baseline levels of the original DAT-SPECT image taken on 02/2013.
In summary, it appears that acetyl-DL-leucine treatment helped to reverse the reduction in DAT binding (as well as reducing RDB symptoms).
And what makes this result intriguing is that the investigators observed a similar effect in Patient #2.
Below is their timeline of assessments and while there have only been two DAT-SPECT imaging sessions to date (one before treatment and one 18 months after initiating acetyl-DL-leucine), there are higher DAT binding intensity scores in the graph below, indicating more dopamine transporter protein is present in the putamen.
 Source: PMC
Source: PMC
|
# # RECAP #2: A small pilot study of two individuals with RBD finds that treatment with a modified leucine amino acid called Acetyl-DL-leucine has positive benefits in terms of reducing the severity of RBD symptoms. Acetyl-DL-leucine also improved dopamine transporter binding in the brains of both individuals, according to brain imaging assessments. # # |
Sounds really interesting, right?
Yes, and the researchers suggest in the discussion of their report that “The stabilization or improvement of DAT-SPECT binding ratios in ADLL-treated patients suggests that treatment might have “rescued” nigrostriatal neuronal somata and/or axons” and they add that the “results support further explorations whether ADLL may have disease-modifying properties in prodromal PD“.
And this result alone would be compelling, but the investigators also saw improvements in a second brain imaging technique.
Another DAT-SPECT-like imaging technique?
No, the second brain imaging technique was Fludeoxyglucose F18 positron emission tomography (FDG-PET).
Flu-de-oxy-what?
Positron emission tomography (or PET) is a commonly used nuclear medicine technique in medicine, where a radioactive (unstable) substance is injected into the body and can be used to visualise different parts/functions of the body.
 The priniciples of PET imaging. Source: Priyalearthproject
The priniciples of PET imaging. Source: Priyalearthproject
By attached radioactive substances to specific molecules, investigators can identify where in the body those molecules are and whether the amount/distribution differs from the norm.
Fludeoxyglucose F18 (or FDG) is basically radioactive sugar. It acts in a very similar manner to glucose – being taken up by cells in need of glucose – and in this fashion it can be used to assess where glucose is being utilised in the body. Such measurements can be used to assess changes in the metabolic activity of the body. For example, given that cancer tumors are high consumers of glucose, FDG is often used to determine where in the body a tumor might be (as in the image below)
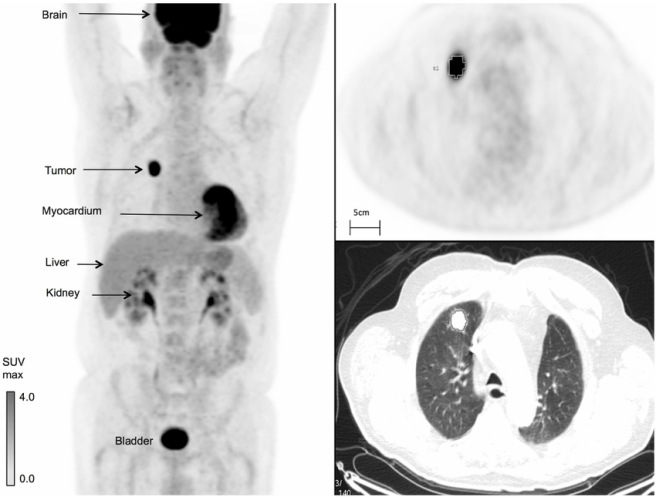 FDG imaging of a cancer tumor in the body. Source: Researchgate
FDG imaging of a cancer tumor in the body. Source: Researchgate
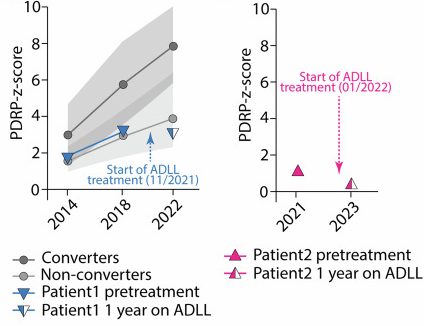
Using FDG PET, scientist have previously generated what is called the “Parkinson’s specific disease-related spatial covariance pattern” (or PDRP) of regional brain glucose metabolism. Some areas demonstrating reduced metabolism, while other regions exhibiting increased metabolism.
 PDRP. Source: Researchgate
PDRP. Source: Researchgate
There is evidence to suggest that the pattern of the PDRP network can be used to distinguish Parkinson’s patients from unaffected healthy controls (Click here to read more about this), and as such it may be able to be used to identify individuals with RBD that are progressing towards Parkinson’s.
In the acetyl-DL-leucine report we are reviewing today, the researchers used FDG-PET and PDRP analysis on the two participants and found that both showed improved scores (a reduction in PDRP-z-score) following the initiation of acetyl-DL-leucine treatment, as shown in the graphs below:
 Source: PMC
Source: PMC
That’s even more encouraging, right?
Yes, but at present the results are coming from a very small study of just two participants and needs to be more carefully evaluated and independently replicated in a much larger study.
Plus, there is one detail in the results that is rather concerning.
What’s is it?
In the report, the authors note that Patient #1 “developed a mild cognitive impairment during the study“.
And you can see this in the graph below.
The graph shows the dates of assessments across the bottom and the scores in the Montreal Cognitive Assessment (or MoCA) and the olfactory function test (TDI) sum-score up the left hand side of the graph while the UPDRS I-III score is provided up the right hand side of the graph. Now if you focus in on the green line across the top of the graph – this is the MoCA cognitive score. It is relatively stable across most of the assessments (between May 2014 to November 2021), but then starts to trend down after the acetyl-DL-leucine treatment is initiated:
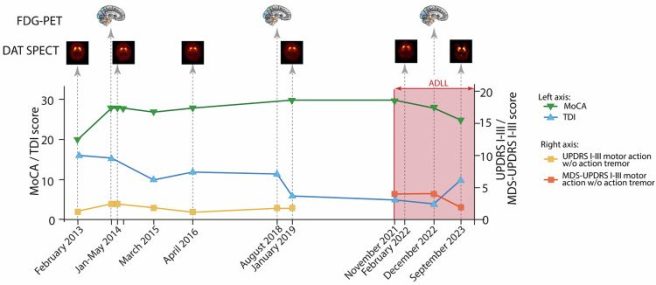 Patient #1 results. Source: PMC
Patient #1 results. Source: PMC
Between January 2019 and November 2021, Patient #1 scores a perfect 30/30 on the MoCA test. But in December 2022 (13 months after starting the acetyl-DL-leucine treatment), this slips to 28 out of 30, and then falls to 25 out of 30 in September 2023.
A score of 18 – 25 out of 30 on the MoCA indicates mild cognitive impairment (Click here to read more about MoCA scores).
Whether this drop in MoCA score is the result of the acetyl-DL-leucine treatment, or simply a separate feature of RBD that is not influenced by the acetyl-DL-leucine treatment, needs to be further investigated, but it does suggest some caution is required moving forward.
But maybe it’s just Patient #1. What happened to Patient #2?
Good question.
Unfortunately, the MoCA scores of Patient #2 also started trending down after initiation of acetyl-DL-leucine treatment, but not enough for a diagnosis of mild cognitive impairment (see the green line in the graph below):
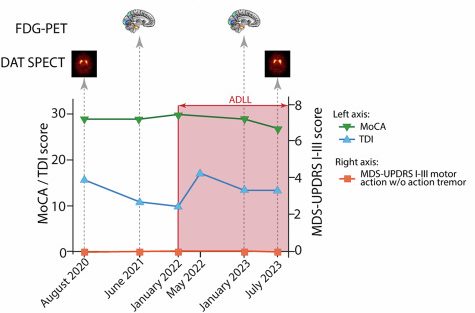 Patient #2 results. Source: PMC
Patient #2 results. Source: PMC
Patient #2 slipped from a score of 30 out of 30 in January 2022 (when acetyl-DL-leucine treatment was initiated) to 29/30 in January 2023, and 27/30 in July 2023. Maybe they were just having a bad day on the day of the assessment – I do not know – but this needs to be investigated in more detail in a much larger study.
Has this ever been observed before?
Not that I am aware of.
Previous studies have found that acetyl-DL-leucine treatment is associated with improved outcomes in executive function and cognition in elderly individuals (Click here and here to read more about this).
|
# # # RECAP #3: Acetyl-DL-leucine treatment improved performance on a second brain imaging technique that measures glucose metabolism. But the treatment was also associated with a reduction in performance on cognitive tests in both study participants. # # # |
Is there going to be a larger study?
This is an important question.
I am not sure if there is a plan for repurposing acetyl-DL-leucine (Tanganil), but there is a biotech firm clinically testing N-acetyl-L-leucine (levacetylleucine).
What is N-acetyl-L-leucine?
Remember when I said that there are two forms acetyl-leucine? (back near the top of this post)
Well, acetyl-DL-leucine (Tanganil) is the version we have been talking about thus far.
And remember when I said that acetyl-DL-leucine is made from the two enantiomers of acetyl-leucine (acetyl-DL-leucine and N-acetyl-L-leucine)?
Well, the biotech firm is IntraBio is developing the N-acetyl-L-leucine (or IB1001) for a variety of conditions.
Why are they focusing on N-acetyl-L-leucine?
In 2020, researchers published this report:
 Title: Acetyl-leucine slows disease progression in lysosomal storage disorders.
Title: Acetyl-leucine slows disease progression in lysosomal storage disorders.
Authors: Kaya E, Smith DA, Smith C, Morris L, Bremova-Ertl T, Cortina-Borja M, Fineran P, Morten KJ, Poulton J, Boland B, Spencer J, Strupp M, Platt FM.
Journal: Brain Commun. 2020 Dec 20;3(1):fcaa148.
PMID: 33738443 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers compared the ability of acetyl-DL-leucine and N-acetyl-L-leucine to help a mouse model of Niemann-Pick disease type C.
What is Niemann-Pick disease type C?
Niemann-Pick disease type C is a rare, inherited, progressive genetic condition that affects multiple organs and systems, including the central nervous system. It is caused by mutations in the NPC1 or NPC2 genes, which prevent the body from properly using cholesterol and other lipids in cells. The condition presents itself as difficulty swallowing, loss of cognitive skills, seizures, and problems with eye movements, walking, and hearing (Click here to read more about this).
In this preclinical study, the researchers breed genetically engineered female mice that do not have a NPC1 gene (these are referred to as Npc1−/− mice). These animals have a 70-90 days life span, with onset of symptoms (gait abnormalities, tremor and weight loss) typically beginning to appear at 40–50 days of age. The investigators began treating the mice with either acetyl-DL-leucine (ADLL), acetyl-L-leucine (ALL), acetyl-D-leucine (ADL), or left the mice untreated (UT) from 55 days of age.
When they looked at the survival of the mice, they found that the ALL treated Npc1−/− mice lived longer than the ADLL treated Npc1−/− mice. As you can see in the graph below, both the ALL treated Npc1−/− mice (Blue line) and the ADLL treated Npc1−/−mice (Green line) lived longer than the untreated Npc1−/−mice (Red line). But importantly, the survival of the ADL treated Npc1−/−mice (Orange line) was no different to that of the untreated mice:
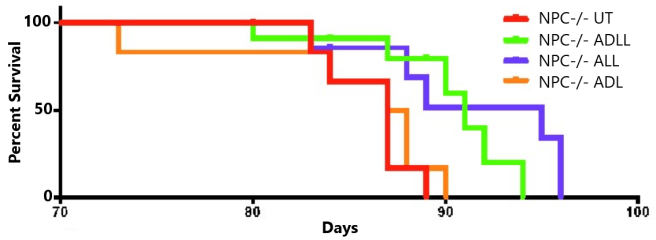 Source: PMC
Source: PMC
The researchers suggested that “these data are consistent with acetyl-L-leucine being the neuroprotective enantiomer“, while acetyl-D-leucine is not active. And this difference between the enantiomer has been found in other contexts (Click here, here and here to read more about this), and the neuroprotective effects of acetyl-L-leucine have been reported by other researchers as well (Click here to read more about this).
So acetyl-L-leucine (and not acetyl-D-leucine) has the neuroprotective effect and this is why the company chose to clinically test acetyl-L-leucine?
Yes.
In the Niemann-Pick disease type C mouse study discussed above (Kaya et al 2020), the researchers also reported improvements in lysosomal function.
What is lysosomal function?
Lysosomes are a key component of the waste disposal/recycling system of our cells.
They are small bags that are full of digestive enzymes that help to break down material inside of cells. Sometimes that material is newly imported from outside of the cell, while other times it may be old proteins that need to be disposed of.
Lysosomes provide the digestive enzymes for the job of breaking down the material.

How lysosomes work. Source: Prezi
We haver discussed lysosomes in previous SoPD posts in more depth – but understand that they are an absolutely critical component of normal biological function inside of cells, and associated with Parkinson’s via multiple genetic risk factors (Click here to read that SoPD post).
Has Intrabio clinically tested acetyl-L-leucine?
Yes, they have.
And in February (2024), IntraBio announced the publication of Phase 3 clinical trial results in the prestigious New England Journal of Medicine:
 Title: Trial of N-Acetyl-l-Leucine in Niemann-Pick Disease Type C.
Title: Trial of N-Acetyl-l-Leucine in Niemann-Pick Disease Type C.
Authors: Bremova-Ertl T, Ramaswami U, Brands M, Foltan T, Gautschi M, Gissen P, Gowing F, Hahn A, Jones S, Kay R, Kolnikova M, Arash-Kaps L, Marquardt T, Mengel E, Park JH, Reichmannová S, Schneider SA, Sivananthan S, Walterfang M, Wibawa P, Strupp M, Martakis K.
Journal: N Engl J Med. 2024 Feb 1;390(5):421-431.
PMID: 38294974 (This report is OPEN ACCESS if you would like to read it)
In this trial, the researchers recruited 60 people with Niemann–Pick disease type C, and randomly assigned them in a 1:1 ratio to receive acetyl-L-leucine for 12 weeks, followed by placebo for 12 weeks, or to receive placebo for 12 weeks, followed by acetyl-L-leucine for 12 weeks. This is what you call a “cross over study”, and the both the participants and the investigators were blind as to who was receiving which treatment. The acetyl-L-leucine treatment or placebo was administered orally two to three times per day across the study.
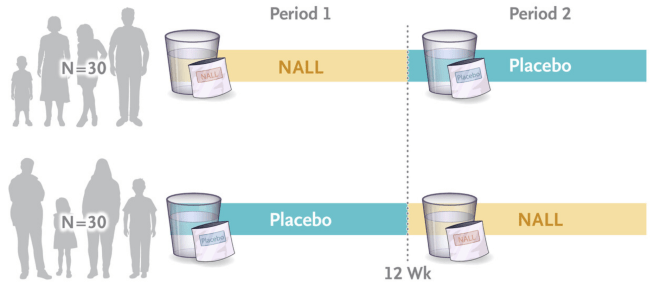 Source: NEJM
Source: NEJM
The primary end point of the study (the predetermined measure of success) was the total score on the Scale for the Assessment and Rating of Ataxia (SARA). This assessment has a range of 0 to 40, with lower scores indicating better outcomes (Click here to read more about this study).
The average change from baseline in the SARA total score after 12 weeks of receiving acetyl-L-leucine was −1.97±2.43 points (indicating improvement in symptoms) and for the placebo group, the average score was −0.60±2.39 points. This difference was statistically significant. You can see in the graphs below that when the participants in the study were on acetyl-L-leucine (the brown lines), most of them had a downward trajectory in their SARA scores (indicating improvement in symptoms), and this balanced out when they were on placebo (blue dashed lines):
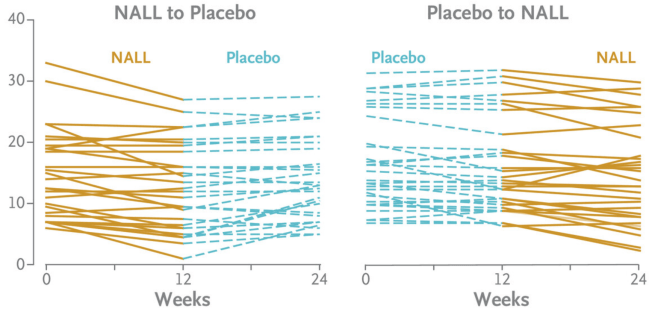 Source: NEJM
Source: NEJM
The investigators also reported that N-acetyl-L-leucine treatment was safe and well tolerated with the incidence of adverse events being similar between N-acetyl-L-leucine and placebo.
Based on these results, the US FDA approved N-Acetyl-L-Leucine/IB1001 (now branded Aqneursa) as a stand-alone therapy for the treatment of Niemann-Pick disease type C (Click here to read more about this).
So rather than testing Acetyl-DL-Leucine in RBD, we should be doing a trial of “Aqneursa” instead?
To be safe, and to hedge one’s bets (learn as much as we can), you would probably want to test both agents (in separate trial arms, comparing each to a placebo control). Firstly to replicate and verify the finds of the small pilot study of Acetyl-DL-Leucine, and secondly to see if N-Acetyl-L-Leucine can provide better results.
And what about Parkinson’s? Has anyone ever looked in Parkinson’s?
In addition to the single Parkinson’s case study (the restless leg individual near the top of this post), there has only been one other study of N-acetyl-L-leucine in the context of Parkinson’s and that was a preclinical study. It found a positive result, but it was in a neurotoxin mouse model of Parkinson’s and the N-acetyl-L-leucine was administered immediately after the toxin – not a great test of neuroprotection (Click here to read more about that study).
Before we can really comment here on whether N-acetyl-L-leucine could be useful in Parkinson’s, we would need more research.
So what does it all mean?
I have thought long and hard about writing a post on this research. Despite being a very small study cohort (only two participants), the results are very interesting (as the investigators noted in a press summary, “what is particularly intriguing is the imaging data, because the improvement in brain pathology cannot be explained by a placebo effect” – click here to read more). In addition, a number of readers have emailed me and asked me about this recent RBD report.
This is the kind of post that puts one in a very tricky position.
But I think it is important to put the research in context, and to highlight any concerns. Firstly, the studies were conducted in individuals with RBD, not Parkinson’s. RBD is considered a risk factor for Parkinson’s, so it could be thought of as a very early stage in the process of this neurodegenerative condition. But in RBD, the majority of the dopamine neurons are likely still present in the brain, so perhaps it is more feasible to restore a reduction of DAT activity in the putamen with some acetyl-DL-leucine treatment. I do not know if this will be the same case for someone diagnosed with Parkinson’s, where the loss of dopamine neurons is greater.
More importantly, as I indicated above, the decline in the cognitive test (MoCA) score during the treatment phase is a big red flag. This will need to be further investigated. It is strange that this agent has been available for decades in France, but this is the first time cognitive issues have been associated with its use. I am not sure if Tanganil is used daily for long periods of time in the treatment of vertigo. As I say, more research is needed.
The results from the N-acetyl-L-leucine studies are very encouraging, particularly for the Niemann-Pick disease type C community and Intrabio is to be congratulated on achieving such a fantastic result and getting their product across the finish line for the patients. It would be interesting to see N-acetyl-L-leucine explored in the context of Parkinson’s, particularly given the lysosomal function connection.
ADDENDUM: A CORRECTION REGARDING THIS POST
Keen eyed reader “ZZ” made an important observation in the comments section below regarding the cognitive impairment concerns I mentioned above.
ZZ noted that in the supplemental files of the Oertel et al paper discussed in this post, there is access to the peer-reviewer comments. The peer-reviewers also expressed concern about the trend towards cognitive decline with N-acetyl-DL-leucine treatment, to which the Oertel and collaborators responded:
” We agree that during the period of ADLL therapy there is a tendency for the MoCA score of patient 1 to worsen. We therefore re-invited (February 2024 – still under ADLL therapy) patient 1 for a new MoCA assessment and the score was 24, which is, by definition of the inclusion criteria, in the range of a mild cognitive impairment. Thus, we cannot rule out that this now 80-year-old RBD patient will develop cognitive impairment in the future. In patient 2 (now 59 years of age), there is also a trend towards lower normal MoCA values during the period of the ADLL therapy. A recent MoCA test (March 2024 – still under ADLL therapy) revealed a score of 28 – this finding is in line with the stated variation in the results of the MoCA screening test”
So perhaps my cognitive impairment concern is slightly overdone. Regardless, it will need to be carefully watched in larger future trials in this space.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The author of this post is an employee of Cure Parkinson’s, so he might be a little bit biased in his views on research and clinical trials supported by the trust. That said, the trust has not requested the production of this post, and the author is sharing it simply because it may be of interest to the Parkinson’s community.
The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from sleepadvisor


Hi Simon,
thank you for addressing this topic! I would like to point out that the peer review file for the “ADLL in RBD” paper can be found online in the Supplementary Information section of the original publication. It includes the following additional details about mild cognitive impairment, as quoted from the author’s response to a referee’s query.
“We agree that during the period of ADLL therapy there is a tendency for the MoCA score of patient 1 to worsen. We therefore reinvited (February 2024 – still under ADLL therapy) patient 1 for a new MoCA assessment and the score was 24, which is, by definition of the inclusion criteria, in the range of a mild cognitive impairment. Thus, we cannot rule out that this now 80-year-old RBD patient will develop cognitive impairment in the future. In patient 2 (now 59 years of age), there is also a trend towards lower normal MoCA values during the period of the ADLL therapy. A recent MoCA test (March 2024 – still under ADLL therapy) revealed a score of 28 – this finding is in line with the stated variation in the results of the MoCA screening test.”
Best wishes,
zz
LikeLiked by 1 person
Well spotted ZZ! Bravo indeed. I missed that completely. That is reassuring to know. Thank you so much for sharing your observation. I will make a note of that later tonight on the post.
Kind regards,
Simon
LikeLiked by 1 person
“It is strange that this agent has been available for decades in France, but this is the first time cognitive issues have been associated with its use. I am not sure if Tanganil is used daily for long periods of time in the treatment of vertigo”
In the RBD case reports, a much higher dosage was used than in the treatment of vertigo – 5 G versus 1 G respectively.
Also, the cognitive impairments may be due to the non-therapeutic D enantiomer. That sort of issue turned out to be the cause of the teratogenic effects of Thalidomide.
LikeLike
Hi ParkBear,
Thanks for your comment.
Yeah, I was wondering if the D enantiomer was not as “inactive” as the preclinical data suggests. But reader ZZ has just pointed out that the peer reviewer notes for this report are available in the supplementary notes and they address the cognitive issue, noting that patient #2’s MoCA score improved more recently (see elsewhere in the comments here).
Kind regards,
Simon
LikeLiked by 1 person
“In the RBD case reports, a much higher dosage was used than in the treatment of vertigo – 5 G versus 1 G respectively.”
This is incorrect: https://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?typedoc=N&specid=68276457
“La dose recommandée est de 1,5 g à 2 g par jour, soit 3 à 4 comprimés en deux prises matin et soir. […] La durée du traitement est variable selon l’évolution clinique (de 10 jours à 5 ou 6 semaines); au début du traitement ou en cas d’insuccès, la posologie peut être augmentée à 3 g voire 4 g par jour, soit 6 à 8 comprimés par jour.”
So, for non-French speakers, Tanganil (DL form) has been used in France (and some other former French colonies such as Vietnam, Lebanon and Tunisia) for more than 65 years at 1.5 to 4 g/day for 10 days to 6 weeks. So the 5g dose in this RBD study is not “much higher”. That being said, as tanganil is available OTC, it’s hard to know how much people use in practice. Despite what the box says, it’s possible that most users only take one pill a day (500 mg). But surely over 65 years, some users took (on purpose or inadvertently) 4 g per day over a few weeks, and maybe even larger doses. A following rapid cognitive decline would hopefully have been detected in surveillance databases.
LikeLiked by 1 person
I thought that the lack of signal for cognitive impairment in French data might be due to the fact that it was either rarely used or only used OTC. But looking at the data ( https://www.data.gouv.fr/fr/datasets/open-medic-base-complete-sur-les-depenses-de-medicaments-interregimes ) acetylleucine (ATC code N07CA04) is the 99th most prescribed drugs in France with about 5 million boxes prescribed per year. This number has been constant from 2015 to today (I couldn’t find data before). 70% of these boxes are prescribed to people who are over 60yo (and among these, users are 2/3 females and 1/3 males).
So it’s massively prescribed at the population level, especially among the elderly. And on top of these 5 million yearly prescriptions there is an unknown number of people buying it without a prescription. Maybe as many. So I would assume that in terms of total sale (with or without prescription), acetyl-DL-leucine must be among the top 50 of the most used drugs in France. Every year for 65 years.
However, the data also includes the number of patients (if I understand correctly) and this gives an average of 4 boxes used by patient per year (when prescribed). One box has 30 pills. That’s 60 g of acetyl-DL-leucine per year. That’s way less than the 5*365 = 1,825 g/y in the RBD study. But 60 g/y is an average, some patients must be way above or way below. And again, that’s only the ones getting a prescription.
Surely a signal would have been caught if it caused massive cognitive impairment?!
Also, this means that French researchers could check if acetyl-DL-leucine users have more or less PD than those getting prescribed other anti-vertigo drugs (e.g. bétahistine N07CA01)?
For what it’s worth, tanganil/acétylleucine are discussed a lot on French social media but I checked Twitter, Reddit and on Google and found NOTHING for “tanganil mémoire”, “tanganil trou de mémoire”, “tanganil cognitifs”, etc.
LikeLike
I think there’s a typo here: “and the neuroprotective effects of acetyl-D-leucine have been reported by other researchers as well (Click here to read more about this).” It should be -L-.
Also, I don’t know if this is relevant but in “Drug Interactions”, IntraBio notes:
“Avoid concomitant use of AQNEURSA with N-acetyl-DL-leucine or N-acetyl-D-leucine. The D-enantiomer, N-acetyl-D-leucine, competes with levacetylleucine for monocarboxylate transporter uptake, which may reduce the levacetylleucine efficacy.” (source)
LikeLike
I’ll stop spamming your blog Simon but it’s good to note that Tanganil was failed in MSA-C, another synucleinopathy: Lack of benefit of acetyl-dl-leucine in patients with multiple system atrophy of the cerebellar type 2017.
“The nine treated patients were monitored as outpatients under the therapy with acetyl-DL-leucine at a dosage of 3 g/day (2 × 500 mg tablets three times a day) for the first week, and then 4.5 g/day (3 × 500 mg tablets three times a day). Evaluations were performed at baseline, before the beginning of the treatment (T0), and after 4 (T1) and 12 weeks (T2) (± 5 days) from the start of the therapy with acetyl- DL -leucine.”
“Five patients reported a minimal improvement after 4 weeks at PGIC, and three of them reported further minimal improvement after 12 weeks. Four patients (patients 3, 5, 6 and 9) decided to continue self-medication for N 12 weeks.”
“Two patients discontinued the treatment, one because of lack of improvement, the other because of side effects (i.e., muscle stiffness, numbness and impaired gait, which disappeared after treatment interruption). None of the other patients reported side effects.”
“In conclusion, we observed no significant benefit on the functional motor disability, i.e. unsteadiness and finger dexterity, in a small, homogeneous cohort of MSA-C patients treated with acetyl- DL -leucine for 12 weeks. The slight and temporary subjective improvement could be due to a placebo effect. Our observations discourage larger, well-designed studies to assess acetyl- DL -leucine as a symptomatic therapy for MSA-C patients, despite the limitations of the study, such as the small sample size and absence of a control group. Our results are not generalisable to other forms of degenerative cerebellar ataxia. Different pathomechanisms and involvement of different neurological subsystems, like in MSA-C, may lead to contradictory results when heterogeneous cohorts of ataxic patients are assessed in clinical trials”
LikeLike
Interesting paper: Small Molecule, Big Hope-Can Acetyl-DL-Leucine Reverse Parkinson’s Disease? 2024
In PPMI, those who had RBD at baseline but later reversed it “showed the fastest motor progression” and “the most severe gray matter atrophy in the middle frontal gyrus” (Evolution patterns of probable REM sleep behavior disorder predicts Parkinson’s disease progression 2022).
Leucine might interact with DAT binding (Molecular dynamics of leucine and dopamine transporter proteins in a model cell membrane lipid bilayer 2010).
LikeLike