|
# # # # At the end of each year, it is a useful process to take stock and review what we have learnt over the last 12 months. 2024 has been an important year for Parkinson’s research, with a lot of clinical trial results being reported and new insights being made. In today’s post, we will consider three big Parkinson’s-related research takeaways of 2024 (based on our humble opinions here at the SoPD), and then we will provide an extended overview of some of the important pieces of news from the last 12 months (Be warned: this is a rather long post). # # # # |
 Source: Freepik
Source: Freepik
Science is mostly an iterative process.
A hypothesis is generated and tested. If it is found to be true, new hypotheses are spawned and tested. And brick-by-brick, the foundation of our knowledge grows.
To the outside observer, it must feel like a slow and cumbersome process. But each step needs to be built on a level of certainty. As Sir Prof John Hardy once said “I don’t care if I’m right or if I’m wrong, I simply want to be certain“
 John Hardy. Source: Breakthrough
John Hardy. Source: Breakthrough
In Parkinson’s research, 2024 felt like a year in which we were looking for certainty across many different areas of activity. Regulators were looking for certainty with new therapeutics before they could be approved (the continuous levodopa delivery system called Produodopa – is a good example of this – click here to read an SoPD post on this topic). Researchers sought certainty through independent replication of previous findings (the data on DOPA decarboxylase as a new biomarkers for Parkinson’s is a good example of this – click here to read a previous SoPD post on this topic).
In addition, clinical trialists were looking for certainty regarding new experimental therapies. A number of new cell replacement therapy trials were initiated (the Aspen Neuroscience ASPIRO study is a good example here) and late stage small molecules studies (such as the GLP-1 receptor agonists and alpha synuclein trials – discussed below) gave answers and raised new questions.
2024 was an extremely eventful year for Parkinson’s research.
Below is a list of some of the more interesting Parkinson’s research findings of the year – by month, but starting with the top three according to the team here at SoPD HQ.
|
# EDITOR’S NOTE: The author of this blog is the director of research at the medical research charity Cure Parkinson’s. For the purpose of transparency and to eliminate any sense of bias, where Cure Parkinson’s is a funder of the research it shall be noted. The selection of research topics below are based on his opinion alone and do not reflect the thoughts of any other parties. # |
The 3 main SOPD highlights in Parkinson’s-related research for 2024
(in no particular order – just our opinion)
1. The GLP-1 receptor agonist trial results:
GLP-1 receptor agonists are a class of diabetes drugs that are being repurposed for.. well, almost everything it seems. They are widely used now for weight-loss and obesity, and new data suggests that they could potentially be game-changers across a spectrum of cardiovascular–kidney–metabolic conditions. They are also being clinically tested in neurodegenerative conditions like Alzheimer’s and Parkinson’s.
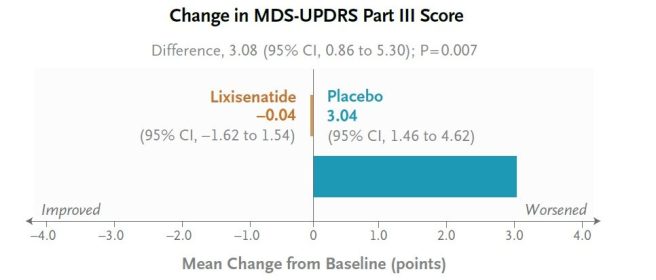
Clinical trial results for Parkinson’s up to 2024 had been encouraging (Click here to read a previous SoPD post on this topic), but 2024 turned out to be something of a rollercoaster ride of additional results. In April, the results of the LIXIPARK study provided further data suggesting that this class of drugs is doing something interesting in people with Parkinson’s. It was a Phase 2 clinical study assessing the GLP-1 receptor agonist Lixisenatide (vs placebo) in 156 people with Parkinson’s.
The study – conducted in France across more than 20 research centers – involved 156 participants being randomised to either lixisenatide (n=78) or to placebo (n=78) and being treated for 12 months. The average baseline MDS-UPDRS part III score in both groups was 15 points. At 12 months, the scores had changed by −0.04 points (indicating improvement in symptoms) in the lixisenatide group vs +3.04 points (indicating worsening) in the placebo. This was a statistically significant positive result and the finding were published in the New England Journal of Medicine (Click here to read more about this). Please note that Cure Parkinson’s was a funder of this trial, and the author of this blog is listed among the authors on the journal paper.
But then on the 14th October, the clinical trial team in charge of the Exenatide Phase 3 study shared the results of their study with the participants. Exenatide is a GLP-1 receptor agonist like lixisenatide. Like lixisenatide, exenatide had given positive Phase 2 stud results (Click here to read a previous SoPD post on that topic), which had led to the 2 years of treatment, 200 participants Phase 3 trial to assess efficacy.
When the investigators pulled the results of the Phase 3 study together, they found that the study had not met its primary endpoint – there was no significant difference in the progression of motor symptoms between the treatment and placebo groups (Click here to read more about this). We are now waiting to see the full results of the study, which should be published soon and then there will be an opportunity to re-assess the future of GLP-1 receptor agonist research in Parkinson’s.
2. Major progress on biomarker research:
As the world settled in for the end of year festive season, a journal paper was published that highlighted a new technique that allows researchers to analyse the contents of lysosomes from blood samples (Click here to read that paper). Lysosomes are tiny sacks of rubbish and enzymes within cells, and they are critical to the waste disposal system of cells. The ability to analyse the contents of these microscopic structures could be extremely significant for Parkinson’s as many forms of the condition are associated with lysosomal function. This could represent a major new biomarker tool for the field.
2024 was an amazing year for biomarker research for Parkinson’s. In addition to the lysosomal work, there was enormous progress made on other biomarkers for Parkinson’s, such as the alpha synuclein seeding assay (which was employed in new biological staging systems for Parkinson’s progress – click here to read an SoPD post on this topic), DOPA decarboxylase levels in cerebrospinal fluid (Click here to read an SoPD post on this topic), and mitochondrial DNA damage (Click here to read an SoPD post on this topic).
There was also significant efforts in the background to pool all of this biomarker research together and provide richer datasets for the broader Parkinson’s research community. A good example of this was the public-private partnership “Accelerating Medicines Partnership in Parkinson’s Disease and Related Disorders” (or AMP PDRD), which was announced by the FNIH on July 17. It builds on an earlier effort, called AMP PD, to identify biomarkers associated with the condition. All of this work will hopefully allow for better diagnosis for the Parkinson’s community, as well as improving the stratification of any subtypes and tracking of disease progression in clinical trials, as well as the development of more targeted therapies.
3. A bit of ADLL for your RBD:
Every now and then, a report comes across the desk here at SoPD HQ that makes one sit up and stare off into the distance for a moment, before one then scratches one’s head and starts to dig deeper into the results. Such a report was published in September, and to be fair I am still scratching my head.
The researchers involved conducted an “exploration of whether Acetyl-DL-leucine may have disease-modifying properties in prodromal Parkinson’s“. Acetyl-DL-leucine (also known as Tanganil) is used for the treatment of vertigo in France, and the researchers gave it to two individuals with REM sleep behaviour disorder (or RBD). RBD involves physically acting our one’s dreams while sleeping, and it is considered an early sign of Parkinson’s (it appears before formal diagnosis in a period of time known as prodromal Parkinson’s).
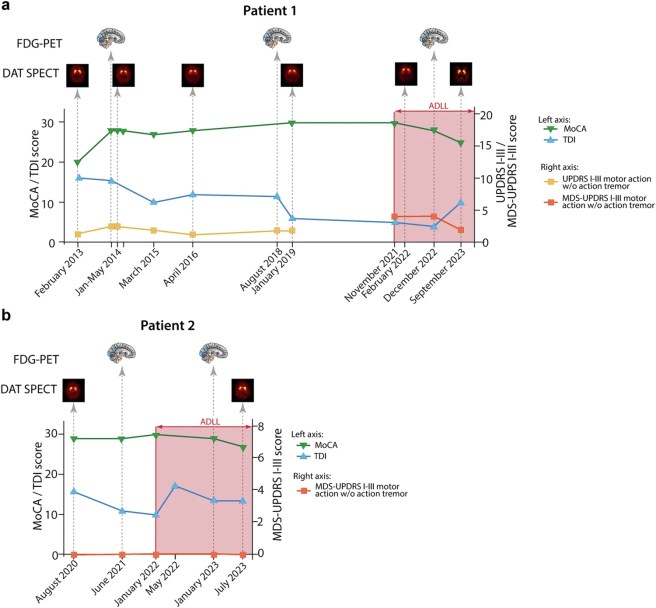
The investigators treated these two individuals with Acetyl-DL-leucine for three weeks and found that RBD-severity sum-scores (RBD-SS-3) decreased in both individuals and their level of RBD symptoms remained reduced for over 18 months of continuous treatment. But most remarkably, the scientists also saw a reversal in DAT-SPECT imaging after 22 months of Acetyl-DL-leucine treatment (Click here to read more about this).
The one worrying detail in the data was that both individuals exhibited a trend towards worsening of cognitive ability (as determined by the Montreal Cognitive Assessment (or MoCA) score – see the green line in the graphs below following the initiation of Acetyl-DL-leucine (ADLL) treatment on the right side of the graphs):
The researchers note that Patient #1 “developed a mild cognitive impairment during the study“. Whether this is the result of the treatment, or simply a separate feature of RBD, needs to be further investigated, but it does suggest some caution is required moving forward. In addition, it is important to remember that this was a very small study involving only two participants. Independent replication in a larger cohort will be required and a better understanding of the mechanism of action is necessary. Whether Acetyl-DL-leucine has any potential with Parkinson’s also needs to be determined, but the findings (particularly the DAT-SPECT results) of this study made it one of the reports that I found most interesting this year, and I look forward to seeing the follow up research.
# # # # # # #
The three pieces of research news above were what grabbed my attention the most in 2024, but it was a very full year of new data and findings. Below, we’ll go month-by-month and discuss some of the other highlights.
Let’s begin with:
1. Novel staging systems for “Parkinson’s”:
“Parkinson’s & dementia with Lewy bodies are currently defined by their clinical features”, but now researchers propose a biological definition of neuronal α-synuclein disease, pushing us “towards an integrated staging system for research” – Two papers were published back-to-back in The Lancet Neurology journal, one proposing the ‘Neuronal Synuclein Disease Integrated Staging System’ (NSD-ISS) and the other proposing the three-component “SynNeurGe” (pronounced ‘synergy’) system.
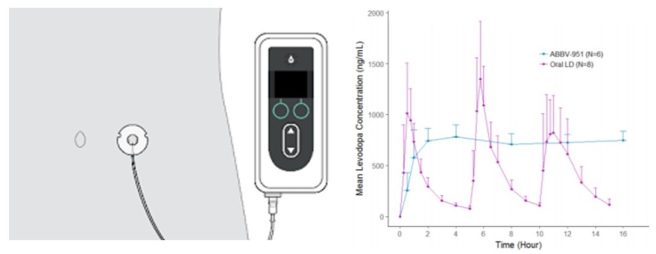
2. The launch of Produodopa:
AbbVie launches Produodopa® (foslevodopa/foscarbidopa) for people living with advanced Parkinson’s in the European Union. This is the first-and-only subcutaneous 24-hour infusion of levodopa-based therapy for the treatment of severe motor fluctuations in Parkinson’s. Rather than having spikes in levodopa levels (due to periodic administration of L-dopa pills – see purple line in the graph below), Produodopa provides a continuous and steady delivery of levodopa (see the blue line in the graph below – click here to read more about this).
3. Robotic apparel for freezing of gait:
Researchers presented a soft robotic apparel that augments hip flexion to avert freezing of gait in Parkinson’s. They conduct an N-of-1 trial in a 73-year-old male with Parkinson’s, involving 5 repeated measurements spanning 6 months. “The wearable garment uses cable-driven actuators & sensors, generating assistive moments in concert with biological muscles”. The positive impact on “FoG-targeting effects were repeatable across multiple days, provoking conditions & environment contexts” (Click here to read more about this and click here and here to read a press summary about the report).
1. The importance of exercise:
It is a small study (only 13 people with Parkinson’s), but 6 months of high-intensity interval training was found to result in an increase in dopamine activity as assessed by brain imaging (“a consistent increase in available DAT sites in the substantia nigra. A more variable increase was observed in available DAT sites in the putamen”), demonstrating the importance of exercise is combating Parkinson’s (Click here to read more about this).
2. The results of the NIC-PD (Nicotine patch) study were published:
The NIC-PD clinical trial involved one-year of transdermal nicotine (or placebo) treatment in 163 people recently diagnosed with Parkinson’s. The researchers found that the treatment did not slow progression in early Parkinson’s. In fact, UPDRS I-III worsened 6.0 in the nicotine group (compared to 3.5 in the placebo; P=0.06). This result suggests that while smoking/nicotine use might reduce one’s risk of developing Parkinson’s, continued use once diagnosed may have a negative impact on progression – click here to read more about this, click here to read an editorial, and click here to read an SoPD post on this topic).
3. The benefits of little blue pills:
For men with erectile dysfunction, phosphodiesterase type 5 inhibitor (PDE5i) initiation is associated with a lower risk for Alzheimer. In analysis that involved 269,725 males, researchers found that the association was particularly strong in those most frequently issued prescriptions. There was also an interesting age effect (greater in over 70 yrs). Time to repurpose viagra again? (Click here to read more about this and click here to read a press summary).
1. More mitochondrial DNA damage data:
A new research paper reported that the Parkinson’s-associated LRRK2-G2019S mutations expressed at endogenous (normal) levels is sufficient to cause mitochondrial DNA damage in cells in culture. Treatment with LRRK2 inhibitors (both selective & non-selective) rescued the phenotype (Click here to read more about this). This is a further accumulation of data supporting mitochondrial DNA damage as a biomarker of Parkinson’s (Click here to read a previous SoPD post on this topic).
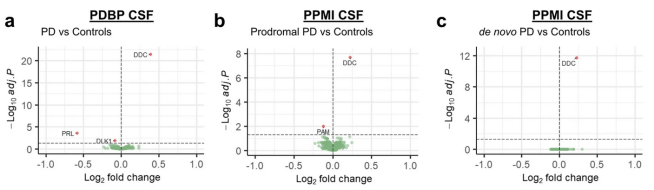
2. More DOPA decarboxylase data:
DOPA decarboxylase (DDC) is an enzyme that is involved in the production of dopamine. Recently data has been accumulating suggesting that it could be a useful biomarker for Parkinson’s. In a very recent study, researchers conducted a comprehensive proteomic analysis of 4877 cerebrospinal fluid, plasma, & urine samples from individuals recently diagnosed with Parkinson’s and they identified DDC as a potential biomarker (Click here to read more about this and click here to read a previous SoPD post on this topic). Look at DDC in the graphs below – way out there all by itself. It’s almost off the chart!
3. New class of drug begins clinical testing for Parkinson’s:
Mission Therapeutics announced the commencement of Phase 1 testing of MTX325 (a CNS penetrant USP30 inhibitor). This is a new class of potentially disease-modifying treatment being tested for Parkinson’s (Click here to read more about this and click here to read a previous SoPD post on this topic).
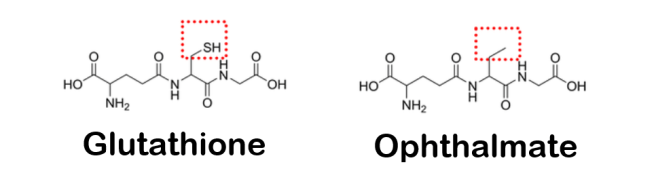
While exploring the inhibition of aromatic L-amino acid decarboxylase (AADC – a crucial enzyme in the production of dopamine) in mice, researchers identified a potential alternative pathway – independent of dopamine signaling – for mediating motor function. They reported that administration of ophthalmate (a tripeptide analog of glutathione) directly to the brain was able to rescue the motor deficits observed in a mouse model of Parkinson’s. They also found that the mechanism of action appears to depend on calcium-sensing receptors (Click here to read more about this and click here to read an SoPD post on this topic).
2. Mitochondrial stratification of Parkinson’s
A new paper proposes that idiopathic Parkinson’s can be stratified according to the severity of mitochondrial respiratory complex I deficiency in neurons. The reporting researchers find 2 emerging disease subtypes with distinct molecular & clinical profiles (Click here to read more about this).
3. Gain therapeutics completes Phase 1 testing:
First hurdle cleared: Gain Therapeutics announced positive results from the single ascending dose (SAD) part of the Phase 1 clinical trial of their novel GCase-targeting small molecule, GT-02287, which is being developed for GBA1-associated Parkinson’s (Click here to read more about this).
1. The radio controlled mouse:
Researchers in Canada presented new data in which they show that activation of specific neurons in the cuneiform or pedunculopontine nuclei in the brain results in locomotion in mice. They also demonstrated that activation of a different set of neurons in the cuneiform nucleus stops mice from moving. What about turning, you ask? The scientists reported that activation of a particular set of neurons in the pedunculopontine nucleus led to ipsilateral turning. All of these neurons are dopamine sensitive, which has implications for Parkinson’s. The videos are quite striking (Click here to read more about this).
2. Subtyping Parkinson’s: OXPHOS-PRS
New research found that a collection of common genetic variations associated with mitochondrial function (collectively named “OXPHOS-PRS”) may “provide a precision medicine tool to stratify idiopathic Parkinson’s patients into a pathogenic subgroup genetically defined by specific mitochondrial impairment”. The researchers used OXPHOS-PRS to re-analyse data from the UP (UDCA in Parkinson’s) study and they found that high OXPHOS-PRS responded more effectively to the treatment (Click here to read more about this).
3. Caffeine and Parkinson’s – new data:
Caffeine intake has long been associated with a reduced risk of Parkinson’s. In a new cross-sectional & longitudinal study, researchers explored the effects of dietary caffeine intake on striatal dopamine function & clinical symptoms in Parkinson’s. The study involved 163 people with recently diagnosed Parkinson’s and 40 controls volunteers who were followed up for 6 years. The researchers found that higher caffeine intake resulted in reduced DAT levels (Click here to read more about this).
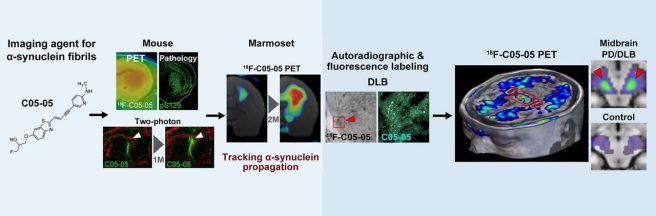
1. New tool for the brain imaging of α-synuclein:
Japanese researchers introduced C05-05, a small-molecule ligand for brain imaging α-synuclein pathologies in animal models and patients with Parkinson’s & related diseases; PET-detectable signals were intensified in the midbrains of Parkinson’s & Dementia with Lewy Body patients (vs controls – click here to read more about this).
2. DOPA-decarboxylase…. AGAIN!
More data was published on the potential of DOPA-decarboxylase levels as a useful tool for Parkinson’s. Researchers provided further support for cerebrospinal fluid (but NOT serum) DOPA decarboxylase levels as a strong potential biomarker for Parkinson’s. Look at the graph below and see how far off the chart and alone DDC is across three different comparisons (Click here to read more about this).
3. The measuring of executive functioning decline in Parkinson’s:
Researchers presented a sensitive new measure of executive functioning decline in PD, called the Parkinson’s Disease Composite of Executive Functioning (or PaCEF). This will be extremely useful not only in the clinical assessment setting, but also in future clinical trials where such tools have been lacking (Click here to read more about this).
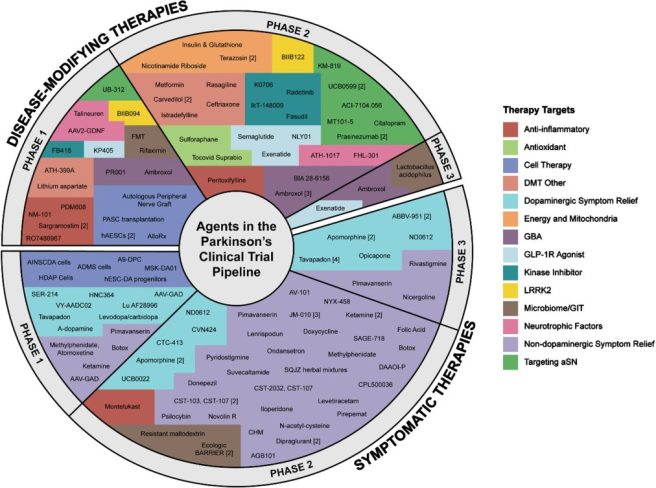
1. The Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline report:
The 2024 report on Parkinson’s drug therapies in the clinical trial pipeline was published. There were 136 active Phase 1–3 trials evaluating drug therapies for PD, of which 76 (56%) were classified as symptomatic trials and 60 (44%) were designated disease modifying. And oooh, that’s a nice pie chart, instead of that ugly bulls eye from previous years. Please note that this report is a Cure Parkinson’s initiative (Click here to read more about this).
2. More research on Camp Lejeune:
A new study explored exposure to contaminated water at Camp Lejeune and Parkinson’s progression. The researchers found “a higher risk of psychosis, falling, & fracture in a cohort of veterans with Parkinson’s who were exposed to trichloroethylene & other volatile organic compounds” (Click here to read more about this).
3. The PD GENEration genetic study of Parkinson’s:
Interesting data from the Parkinson’s Foundation’s PD GENEration study: Researchers report on the ongoing multicentre, observational study, involving 10,510 Parkinson’s participants across more than 85 research centers. 13% of cases=genetic variant; GBA1 variants in 7.7% of all participants, LRRK2=2.4%, PRKN=2.1%, SNCA=0.1%, & PINK1, PARK7 or VPS35=0.2%. Results observed in up to 18% in people with risk factors such as early age at onset, high-risk ancestry or an affected 1st degree relative, but also 9% in people with no risk factors (Click here to read more about this).
1. Levodopa… for Alzheimer’s?:
Chemogenetic activation of dopamine release from VTA neurons increases levels & activity of β-amyloid-degrading enzyme neprilysin, which reduces β-amyloid deposits in the prefrontal cortex (in a neprilysin-dependent manner); Levodopa treatment increases neprilysin, reduces β-amyloid deposits & improves cognition in APP NL-F mice (Click here to read more about this).
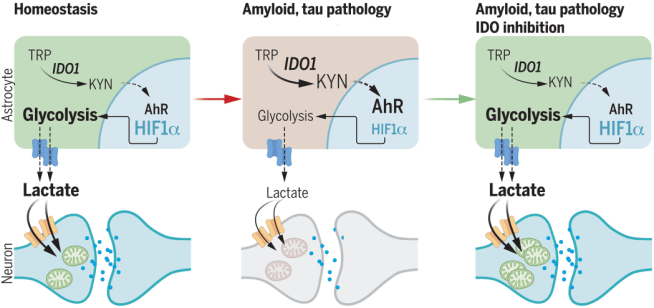
2. Inhibition of IDO1:
Researchers report inhibition of indoleamine-2,3-dioxygenase 1 (IDO1) & production of kynurenine rescues hippocampal synaptic plasticity & memory function in models of amyloid & tau pathology (via astrocytic metabolic support of neurons – click here to read more about this and click here to read press summary on this research).
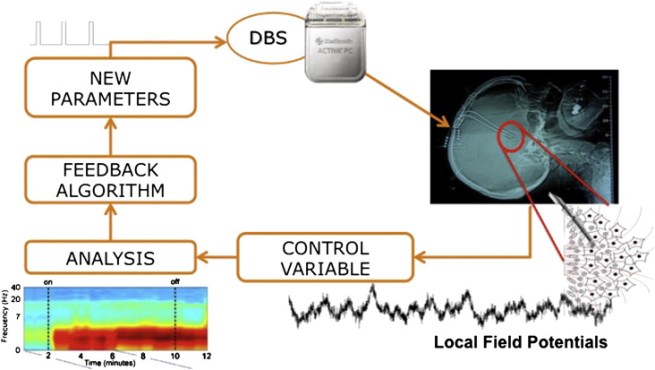
3. First Adaptive deep brain stimulation clinical trial results:
The age of personalised deep brain stimulation is fast approaching: Researchers present the results of a blinded randomized feasibility trial of chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson’s (Click here to read more about this and click here and here to read press summaries on this research).
1. Crhbp for REM:
Researchers reported that Corticotropin Releasing Hormone Binding Protein (Crhbp)+ neurons, in a region of the brain called the pontine sublaterodorsal tegmentum, promote REM sleep (via Nos1+ neurons in the medulla). They noted that loss of these Crhbp+ neurons in mice resulted in less REM sleep. In addition, they analysed postmortem brains of Parkinson’s patients with REM sleep behaviour disorder and they found that CRHBP+ neurons were largely reduced and those remaining contained aggregated α-synuclein (Click here to read more about this).
2. More diabetes medication data:
Another day, another report suggesting sodium-glucose cotransporter 2 (SGLT2) inhibitors decreases the risk of Parkinson’s in people with diabetes (also reduces the risk of dementia); Korean National Health Insurance Service Database, N=1.3M (Click here to read more about this).
3. Air pollution and Parkinson’s:
A case-controlled study found that higher levels of PM2.5 & NO2 exposure were associated with increased risk of Parkinson’s; Curiously, increased PM2.5 exposure was associated with increased risk of developing akinetic rigid PD & dyskinesia (Click here to read more about this).
1. Long-term treatment with Prasinezumab :
A new report from the Roche Prasinezumab Study Group presented data indicating a stablisation of Parkinson’s motor progression in the open-label extension arm of the study (out to 4 years; compared to external comparator). Prasinezumab is an alpha synuclein targeting antibody. The placebo group from the original Pasadena study (now referred to as ‘delayed-start’; n = 94) & the ‘early-start’ (both high & low dose groups from the original study; n = 177) were treated and observed over 4 years. Their MDS–UPDRS III OFF scores were 55% below the median PPMI predictions in year 2 and 66% in year 4. The PASADENA study groups had “numerically lower L-dopa equivalent daily dose (LEDD) intake” and “showed a decreased risk of developing balance issues at year 4” at year 4 compared to the PPMI cohort (Click here to read more about this). The ongoing PADOVA study is a Phase IIb, multicenter, randomized, double-blind, placebo-controlled trial evaluating the efficacy & safety of prasinezumab vs placebo in 586 participants with early-stage Parkinson’s. The results are expected early 2025 (Click here to read more about this).
2. Encouraging gene therapy clinical trial results:
MeiraGTx announce top-line data from their AAV-GAD trial of MGT-GAD-025 in Parkinson’s. This was a 6-month, 3-arm, randomized, double-blind study, that demonstrated that the treatment was safe & well tolerated. At 26 weeks post surgery, there was a statistically significant 18-point improvement in UPDRS Part 3 OFF in the high dose group (Click here to read more about this).
3. One for the ladies:
It was previously suggested that the tauopathy inhibitor, davunetide may have sex-dependent efficacy in women with Progressive supranuclear palsy. Now researchers report the results of a second post-hoc analysis indicating efficacy in females with amnestic mild cognitive impairment (12 weeks treatment, N=144; NCT00422981 – click here to read more about this). The previous post hoc analysis of the results from the clinical trial in PSP (NCT01110720) can be found by clicking here.
1. Disruption of gut homeostasis:
New research found that the Parkinson’s drug entacapone (a catechol-O-methyltransferase (COMT) inhibitor) disrupts gut microbiome homoeostasis (via iron sequestration), selecting for iron-scavenging gut bacteria that encode antimicrobial resistance & virulence genes (Click here to read more about this and click here for the editorial associated with this research).
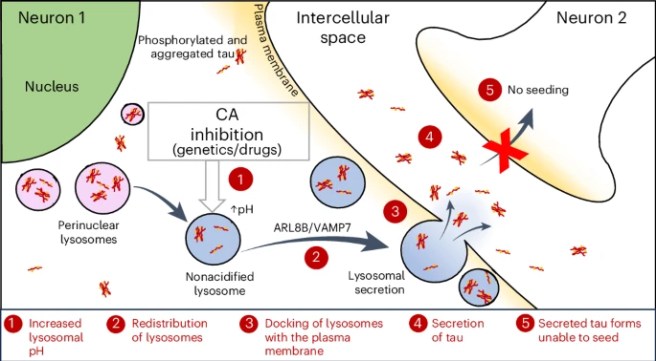
2. Carbonic anhydrase inhibition on Tau:
Researchers reported that carbonic anhydrase inhibition ameliorates Tau toxicity (via enhanced tau secretion). They identified this through a Zebrafish drug screen and CRISPR experiment. Then they tested methazolamide in a mouse model of Alzheimer’s (PS19 tau transgenic mice) and found that it reduced total & phosphorylated tau levels (Click here to read more about this). It is interesting to note here that zonisamide (aka Zonegran – a commonly used Parkinson’s agent) is also a carbonic anhydrase inhibitor.
3. A new mCLAS in sleep:
New paper reports that mouse closed-loop auditory stimulation (mCLAS) is able to boost slow-wave activity during sleep in Parkinson’s (M83) & Alzheimer’s (Tg2576) mice. In Parkinson’s mice, mCLAS significant reduction in NREM sleep consolidation (Click here to read more about this).
1. Prasinezumab continues with new results from PADOVA:
The pharmaceutical company Roche announced that their Phase IIb PADOVA study of the alpha synuclein targeting antibody Prasinezumab just missed its primary endpoint (p=0.0657), but their data suggests possible clinical benefit in early-stage Parkinson’s. This was an 18 month investigation in 586 people with early-stage Parkinson’s (on stable symptomatic treatment). The treatment continued to be well tolerated, with no new safety signals. Positive trends seen across secondary & exploratory endpoints were observed (Click here to read more about this).
2. Functional analysis of the African GBA1 variant:
Researchers (supported by ASAP) published a new paper that reported the functional effect of the African ancestry-specific GBA1 non-coding Parkinsons risk variant that was discovered last year. They discovered that it interferes with splicing of functional GBA1 transcripts and this results in less GCase protein & reduced GCase enzymatic activity (Click here to read more about this).
3. When the Orchestra goes quiet:
The pharmaceutical company UCB (and partner Novartis) announced the results of the proof-of-concept study ORCHESTRA study investigating the efficacy of the small molecule alpha synuclein aggregation inhibitor minzasolmin. They reported that the agent had not reached its primary or secondary endpoints (pre-determined measures of efficacy) in the 18 month study involving 496 patients with early-stage Parkinson’s (Click here to read more about this).
And that is it.
Those were some of the pieces of Parkinson’s research that grabbed our attention here at SoPD HQ in 2024. It is not an exhaustive list, and I apologise to any researchers who feel left out.
It was an extremely eventful year for Parkinson’s research with a lot of clinical trial data being released – some encouraging, while other results providing some clarity and certainty on experimental therapies that have not worked.
Looking ahead, there will be a lot of clinical trial results coming in the new year. And in the next SoPD post – the “Road Ahead” post – we will provide an overview of those studies scheduled to provide results in 2025 and other ongoing/planned studies.
Happy new year to all!
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The author of this post is an employee of Cure Parkinson’s, so he might be a little bit biased in his views on research and clinical trials supported by the trust. That said, the trust has not requested the production of this post, and the author is sharing it simply because it may be of interest to the Parkinson’s community.
The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
In addition, many of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from madisonslibrary

































Happy New Year Simon.
Well done for catching up on the outstanding backlog on monthly and annual reviews for 2024. You must have worked through most of the Christmas festivities.
Thank you once again for all that you do with Science of Parkinson’s. Its really helpful in trying to understand what is going on with this horrid condition.
I think that a better discussion about dementia would help a lot. I suspect that clinicians and researchers are wary of frightening people but reading statements about the high % of PWP having dementia at death is quite scary anyway. Maybe an area for a future series of posts ?
Not that I wan’t to give you extra work
Wishing you a happy, healthy and successful 2025
Eirwen
LikeLiked by 1 person
Hi Eirwen,
Happy new year to you too – I hope all is well. Cognitive impairment is a topic we are exploring in the workshop at CP this year. Need to be very careful and considerate about the language used approaching the topic, but it is an important topic. Under studied in the PD space and little discussed. It will be addressed this year on the SoPD website as well.
Kind regards,
Simon
LikeLike
For Bart’s friend. Let him look at section on glp1 drugs eg exanatide (didn’t reach endpoint in phase 3 studies, whereas fewnch lixenatide trial was effective
Mary Sloper
LikeLike