|
# # # # The diagnostic process for Parkinson’s has been problematic for a long time. Individuals presenting the symptoms often need several clinical evaluations, and confirmation using a brain imaging technique. A biological test for the condition has been lacking and would help tremendously. Recently, however, research (supported by the Michael J Fox Foundation for Parkinson’s research) has indicated that this could be about to change. In today’s post, we will explore recently published research highlighting a new potential biomarker test for Parkinson’s. # # # # |
On the 27th June, 1997, a research report was published in the prestigious scientific journal ‘Science’ that would change the world of Parkinson’s forever.
And I am not exaggerating or overstating here. I know I can sometimes be a little over the top, but the research report in question very much changed the world of Parkinson’s research.
The discovery that tiny variations in a region of DNA that scientists refer to as “alpha synuclein” could increase the risk of developing Parkinson’s gave researchers their first real insights into some of the biology that could potentially be underlying the condition (Click here to read a previous SoPD post on this discovery):

Title: Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease.
Authors: Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL.
Journal: Science. 1997 Jun 27;276(5321):2045-7.
PMID: 9197268
And then – remarkably just two months later – the results of another study were published in the journal ‘Nature’, and these would further cemented alpha synuclein’s place in Parkinson’s research.
In this second research paper, the investigators showed that alpha synuclein was present in “Lewy bodies” – densely packed spheres of protein inside of cells that are one of the characteristic features of the Parkinsonian brain:
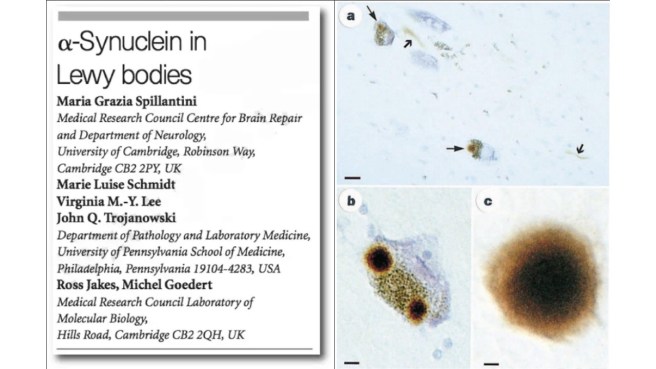 Title: Alpha-synuclein in Lewy bodies.
Title: Alpha-synuclein in Lewy bodies.
Authors: Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M.
Journal: Nature. 1997 Aug 28;388(6645):839-40.
PMID: 9278044
And very suddenly, this poor little protein became public ‘enemy number one’ for the Parkinson’s research community and everyone started digging into the biology associated with it with the hope of finding new avenues for therapeutic intervention and biomarkers for Parkinson’s.
What exactly is alpha synuclein?
It sounds like a distant galaxy, but alpha synuclein is actually one of the most common proteins in our brains. It makes up about 1% of all the protein in a neuron (source).
As a protein, alpha synuclein is mainly located around the synapse of neurons. Synapses are the regions where neurons communicate with other cells. They are often at the tips of the neurons branching arms (called axons). At the synapse, alpha synuclein is known to play important roles in the packaging and transmission of signals between neurons.
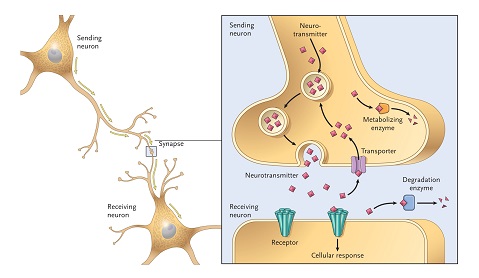 The location of synapses on neurons. Source: Umaryland
The location of synapses on neurons. Source: Umaryland
But years of research have demonstrated that alpha synuclein has other functions in neurons and that it is a very tricky protein to study.
How so?
Well, when alpha synuclein protein is produced by a cell, it normally referred as a ‘natively unfolded protein’. This means that is does not really have a defined structure.
When it is first produced, alpha synuclein protein will look something like this:

Alpha synuclein. Source: Wikipedia
In this form, alpha synuclein is considered a monomer – which is a single molecule that can bind to other molecules. And from this point on, alpha synuclein has lots of different options on what it can do. For example, sometimes it embeds itself in the cell wall (the membrane), while other times it becomes helically folded into a structure called a tetramer.
 Source: Mdpi
Source: Mdpi
Sometimes it binds to other alpha synuclein proteins and they form an oligomer (a collection of a certain number of monomers in a specific repeating structure). In all of these various forms, it is believed that alpha synuclein has certain functions.
In Parkinson’s, however, alpha synuclein appears to “mis-fold” and start clumping (or aggregating) together to form fibrils.

Images of monomers, oligomers and fibrils. Source: Brain
A curious feature of alpha synuclein fibrils is that they can ‘seed’ the aggregation of other alpha synuclein monomers.
What does that mean? Seed?!?
It means that the fibril form of alpha synuclein can connect with and convert the shape of recently made alpha synuclein protein (monomers) to a fibril-causing conformation.
This particular characteristic of fibrils makes them self-propagating. This mean that they can easily make more copies of themselves, by grabbing naïve alpha synuclein monomers and converting them to fibril-prone proteins.
 Source: PMC
Source: PMC
This feature also provides a possible mechanism for how the disease might be spreading within the brain. Researchers have speculated that the disease could be progressing via cell-to-cell transmission of alpha synuclein fibrils. ‘Sick’ cells may be releasing alpha synuclein fibrils out into the world and they could be taken in by ‘healthy’ cells. Once alpha synuclein fibrils are absorbed by a ‘healthy’ cell, the fibrils can start converting the shape of resident alpha synuclein monomers inside that cell, causing them to become fibrils, which ultimately results in more fibrils being produced.
And this may of course go on to cause trouble for the wider brain and body.
What kind of trouble?
Parkinson’s-kind of trouble.
As mentioned at the top of the post, the classical hall mark of the Parkinsonian brain is the clustering of alpha synuclein in specific regions of the brain. So of the regions of the brain may be more vulnerable to this alpha synuclein protein aggregation than others, which may result in the features and symptoms of Parkinson’s.
That’s the theory at least.
So alpha synuclein is the bad guy then?
Well, it depends on who you ask.
Que?
If you put ten Parkinson’s researchers into a room and ask them if alpha synuclein could be a bad actor, you will get 11 different answers (and it’s very likely that someone will get angry and throw a chair).
If this sounds confusing, it is understandable.
Such is the nature of this curious little protein that 20+ years on from the discovery of its association with the PD brain, scientists are still arguing over its true structure(s), function(s), and potential role(s) in Parkinson’s.
BUT, the fact that alpha synuclein monomers bind to alpha synuclein fibrils in a propagating fashion, gave researchers an interesting idea:
Could this characteristic be used as a biomarker for Parkinson’s?
What do you mean?
I mean, if alpha synuclein proteins can seed aggregation in people with Parkinson’s, could this be a biological test for diagnosing people with the condition?
Could there be ‘seeding’ alpha synuclein fibrils circulating in biological fluids of people with Parkinson’s? And if they are there, could we take a blood sample or some cerebrospinal fluid and analyse them for alpha synuclein to see if it has a tendency to aggregate?
And if so, could this be a test for Parkinson’s?
What is cerebrospinal fluid?
Cerebrospinal fluid is the liquid our brains sit in. It can be sampled via a procedure called a lumbar puncture (LP). An LP is performed by inserting a tiny needle between the vertebrae at the bottom of the spinal cord and a small sample of cerebrospinal fluid can be taken.
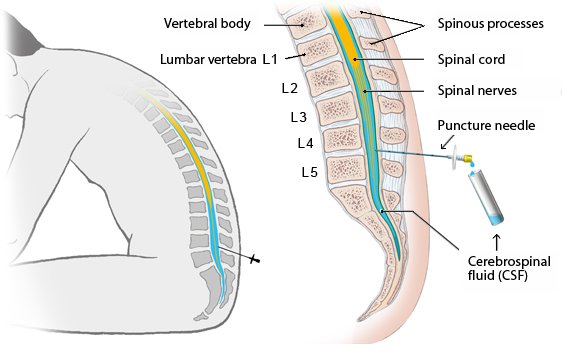 Lumbar puncture. Source: InformedHealth
Lumbar puncture. Source: InformedHealth
Cerebrospinal fluid is an accessible liquid sample that is directly exposed to the brain, so if seeding alpha synuclein fibrils are going to be present, it is the best place to start looking.
Has alpha synuclein protein been detected in cerebrospinal fluid?
Yes it has.
Researchers have even detected oligomeric alpha synuclein in samples of cerebrospinal fluid from people with Parkinson’s (Click here to read more about this), and the levels of oligomeric alpha synuclein have been associated with Parkinson’s dementia when compared with cases of Alzheimer’s or healthy controls (Click here to read more about this).
Ok. So have people explored this idea of seeding alpha synuclein fibrils as a potential biomarker?
Yes, they have. In fact there has been a long history of exploring this idea. And The Michael J Fox Foundation for Parkinson’s Research has been instrumental in driving these efforts.
These efforts started a few years back and the first report suggesting that it could be a viable test was this publication:
 Title: Development of a Biochemical Diagnosis of Parkinson Disease by Detection of α-Synuclein Misfolded Aggregates in Cerebrospinal Fluid.
Title: Development of a Biochemical Diagnosis of Parkinson Disease by Detection of α-Synuclein Misfolded Aggregates in Cerebrospinal Fluid.
Authors: Shahnawaz M, Tokuda T, Waragai M, Mendez N, Ishii R, Trenkwalder C, Mollenhauer B, Soto C.
Journal: JAMA Neurol. 2017 Feb 1;74(2):163-172.
PMID: 27918765 (This report is OPEN ACCESS if you would like to read it)
This first study was a proof-of-concept investigation assessing a technology platform that allows for the extremely sensitive detection of misfolded alpha synuclein aggregates through the cycling amplification of the misfolding and aggregation process.
The platform has two phases: In the first phase, cerebrospinal fluid samples containing minute amounts of misfolded alpha synuclein oligomers is added to a solution containing an excess of monomeric alpha synuclein protein. This mix is incubated in conditions that stimulate ‘seeding’ – the growth of alpha synuclein fibrils. This phase is called elongation.
In the second phase, the samples are subjected to mechanical force (sometimes referred to as quaking or fragmentation) that helps to break down the aggregates, thus increasing the number of potential ‘seeds’. And then the cycle starts again here with another round of elongation.
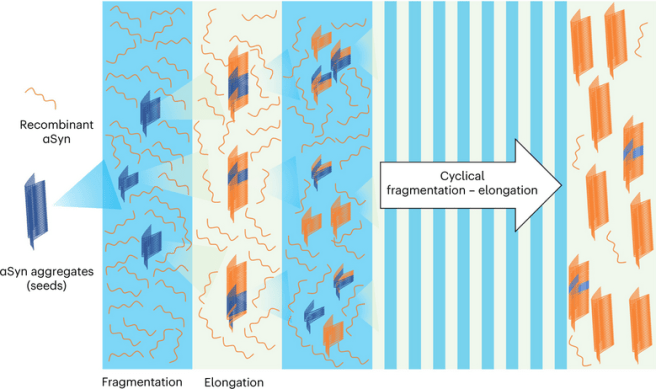 Source: Nature
Source: Nature
In this fashion, if there are any alpha synuclein seeds in the cerebrospinal fluid samples, over time (and successive cycles) they will become amplified, which will clearly indicate their presence. And this provides researchers with a quantitative readout – a potential biomarker test for Parkinson’s.
This first study involved cerebrospinal fluid samples from 76 patients diagnosed with Parkinson’s. They also assessed 10 samples from individuals with Dementia with Lewy bodies and 10 samples for individuals affected by multiple system atrophy (MSA) – both conditions are similar to Parkinson’s.
For control samples, they included cerebrospinal fluid samples from 83 peoples with other neurologic diseases, 2 people without any neurologic conditions, and 14 patients diagnosed with Alzheimer’s.
So what did they find in their analysis?
The researchers firstly tested the platform with different amounts of alpha synuclein seeding-prone oligomers and found that the readout signal was directly proportional to the amount of alpha synuclein oligomers that they added to the reaction. This was very pleasing and allowed the researchers to next blindly test the cerebrospinal fluid samples collected from their volunteers.
After running those samples through the platform, the scientists found that they could correctly identify the patients affected by Parkinson’s with an overall sensitivity of 88.5% and specificity of 96.9%.
What do you mean by “sensitivity of 88.5% and specificity of 96.9%”?
Sensitivity (think: true positive rate) relates to the probability of a positive test result. A highly ‘sensitive’ test means that there are few false negative results, and thus fewer cases of disease are missed. Thus, 88.5% is quite high indicating the test is useful, whereas a 50% test would be no better than chance or guessing.
And specificity (think: true negative rate) is the probability of a negative test result, based on the individual truly being negative. The specificity of a test is its ability to designate an individual who does not have a disease as negative. A highly specific test means that there are few false positive results. And again, 96.9% is very encouraging in terms of specificity.
Interestingly, all 10 of the DLB samples (100% sensitivity) and 8 (80%) of the MSA samples gave positive results (and similar results have been reported by others – click here to read more about this), indicating that the alpha synuclein seeding assay could be useful in identifying not just Parkinson’s, but synucleinopathies in general.
Synuclei-what?
Synucleinopathies are a group of neurodegenerative disorders in which the protein alpha-synuclein accumulates abnormally. The three most common types of synucleinopathy: Parkinson’s, DLB, and MSA. With all of these conditions being characterised by alpha synuclein accumulation, it is not really surprising that an alpha synuclein assay would give positive results for them all.
It was interesting in this study, however, that PD and DLB provided stronger results than MSA. In their write up of the results, the researchers noted that the platform (in its format at the time) “cannot differentiate PD from other synucleinopathies (eg, MSA and DLB) that also accumulate αSyn aggregates” and they suggested that “it is likely that alpha synuclein aggregates implicated in distinct synucleinopathies may adopt different conformations that could be differentiated after amplification“.
The researchers also concluded that “Further studies are needed to investigate the usefulness of [the platform] to monitor disease progression and for preclinical identification of patients who may develop PD”.
In 2020, the researchers published a new report in which they had analysed samples from a larger cohort of people affected by Parkinson’s and multiple system atrophy (MSA).
Here is the report of that study:
 Title: Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy.
Title: Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy.
Authors: Shahnawaz M, Mukherjee A, Pritzkow S, Mendez N, Rabadia P, Liu X, Hu B, Schmeichel A, Singer W, Wu G, Tsai AL, Shirani H, Nilsson KPR, Low PA, Soto C.
Journal: Nature. 2020 Feb;578(7794):273-277.
PMID: 32025029 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers assessed cerebrospinal fluid samples from 94 people with Parkinson’s, 75 people diagnosed with MSA, and 56 “control” people who were diagnosed with other neurological diseases.
The controls in these studies are all affected by other conditions. Is that normal?
No, usually you want ‘controls’ who are healthy and unaffected by any conditions. But cerebrospinal fluid samples are challenging to collect and usually only taken from people with a medical ailment. So for these first studies, the researchers were using what they had access to.
The researchers found that they clearly differentiate between the three groups of samples being analysed, with the Parkinson’s samples having higher levels of seeding aggregation than the MSA and an overall sensitivity of 95.4%:
 Source: PMC
Source: PMC
Here the researchers found that “α-synuclein aggregates that are associated with Parkinson’s disease and multiple system atrophy correspond to different conformational strains of α-synuclein, which can be amplified and detected“. It is unfortunate that DLB samples were not included in this more thorough analysis, but the researchers next turned that attention to another condition closely associated with Parkinson’s: SWEDDs.
This report presents the results of that study:
 Title: Seed Amplification Assay to Diagnose Early Parkinson’s and Predict Dopaminergic Deficit Progression.
Title: Seed Amplification Assay to Diagnose Early Parkinson’s and Predict Dopaminergic Deficit Progression.
Authors: Concha-Marambio L, Farris CM, Holguin B, Ma Y, Seibyl J, Russo MJ, Kang UJ, Hutten SJ, Merchant K, Shahnawaz M, Soto C.
Journal: Mov Disord. 2021 Oct;36(10):2444-2446.
PMID: 34236720 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers applied the same platform to samples from 30 people with Parkinson’s, 30 healthy controls, and 20 SWEDDs.
Sorry, but what are SWEDDs?
SWEDDs are individual who have been clinically diagnosed with Parkinson’s but exhibit “Scans Without Evidence of Dopaminergic Deficit” (or SWEDD).
SWEDD is a non-degenerative variant of the parkinsonian syndromes. They are defined by the lack of clear evidence of neurodegeneration in the dopamine system from brain imaging techniques like DaTSCAN:
 Source: Frontiers
Source: Frontiers
Longitudinal study of SWEDD patients has found that they do not clinically progress significantly, and they are insensitive to treatment with levodopa (Click here to read more about this). Postmortem analyses of the brains of SWEDD cases have found no evidence of alpha synuclein aggregation (Click here to read more about this).
In this study, the researchers also explored analysis of samples over time, because they wanted to know if the results of their platform change over time with the progression of the disease. They found that the alpha synuclein seeding platform displayed 96.2% sensitivity and 96.7% specificity for Parkinson’s when compared to the healthy controls at the start of the study. At a 3‐year follow‐up assessment of new samples, the results had changed very little with 96.4% sensitivity and 93.8% specificity.
When cerebrospinal fluid samples from SWEDDs were compared with Parkinson’s samples on the alpha synuclein seeding platform, the researchers reported that 4 of the SWEDD samples tested positive, 15 negative, and 1 was considered inconclusive. Interestingly, 2 of the 4 positive SWEDD cases later had DaTscans that presented dopamine degeneration consistent with a Parkinson’s diagnosis. This result suggests that the platform may be very useful in a clinical setting in cases with disputable or questionable diagnoses.
In a more recent study, the research assessed their alpha synuclein seeding assay with similar amplification assays developed independently by two other labs, to assess reliability between labs and platforms testing the same samples.
Here is the report of these experiments:
 Title: High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease.
Title: High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease.
Authors: Russo MJ, Orru CD, Concha-Marambio L, Giaisi S, Groveman BR, Farris CM, Holguin B, Hughson AG, LaFontant DE, Caspell-Garcia C, Coffey CS, Mollon J, Hutten SJ, Merchant K, Heym RG, Soto C, Caughey B, Kang UJ.
Journal: Acta Neuropathol Commun. 2021 Nov 6;9(1):179.
PMID: 34742348 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers used cerebrospinal fluid samples from 30 people with Parkinson’s, 30 unaffected controls, and 20 individuals with SWEDD (all part of the Michael J Fox Foundation’s Parkinson’s Progression Markers Initiative (PPMI) study.
The PD and control samples were collected at both baseline and at 3 years follow up assessments (so there were 140 total CSF samples in total – including the 20 SWEDD cases). All of these samples were analysed in parallel by three labs according to their own established and optimized alpha synuclein seeding assay platforms.
The researchers reported results that were “remarkably similar across laboratories, displaying high diagnostic performance (sensitivity ranging from 86 to 96% and specificity from 93 to 100%)“, indicating encouraging reproducibility of the approach.
Has there been a really big test of the alpha synuclein seeding assay platform?
Yes, there has.
Very recently, the same researchers and the Michael J Fox foundation published this report:
 Title: Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study.
Title: Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study.
Authors: Siderowf A, Concha-Marambio L, Lafontant DE, Farris CM, Ma Y, Urenia PA, Nguyen H, Alcalay RN, Chahine LM, Foroud T, Galasko D, Kieburtz K, Merchant K, Mollenhauer B, Poston KL, Seibyl J, Simuni T, Tanner CM, Weintraub D, Videnovic A, Choi SH, Kurth R, Caspell-Garcia C, Coffey CS, Frasier M, Oliveira LMA, Hutten SJ, Sherer T, Marek K, Soto C; Parkinson’s Progression Markers Initiative.
Journal: Lancet Neurol. 2023 May;22(5):407-417.
PMID: 37059509
In this study, the research team behind the alpha synuclein seeding assay platform analysed 1123 samples from 33 academic research centers from around the world (including Austria, Canada, France, Germany, Greece, Israel, Italy, the Netherlands, Norway, Spain, the UK, and the USA). In total, there were:
- 545 cases of Parkinson’s
- 163 unaffected controls
- 54 SWEDD
- 51 individuals displaying prodromal symptoms for Parkinson’s, but not yet diagnosed
- 310 individuals carrying genetic risk factors for Parkinson’s, but not displaying symptoms
Within the Parkinson’s cases, there were 373 with idiopathic (or spontaneous) PD, 123 with LRRK2-associated Parkinson’s, and 49 with GBA1-associated Parkinson’s. Between all of the Parkinson’s cases and unaffected controls the sensitivity was 87.7% and specificity was 96.3%.
The study was very focused on Parkinson’s (no DLB or MSA samples were analysed in this study), but interestingly, the sensitivity for the alpha synuclein seeding assay in the LRRK2-associated Parkinson’s cases fell to 67.5%, while the idiopathic PD and GBA1-associated PD levels remained extremely high (93.3% and 95.9%, respectively).
Why was the sensitivity lower in the LRRK2-associated Parkinson’s samples?
This result is similar to what has been observed in other alpha synuclein seeding studies (Click here to read an example). And it is not surprising as there have been a number of studies examining the postmortem brains of people who passed away with LRRK2-associated Parkinson’s, and they have reported an absence of Lewy bodies in a significant subset of cases (Click here to read more about this).
When the researchers looked at the clinical symptoms of those with positive and negative seeding results within the LRRK2-associated Parkinson’s cohort, there were some interesting differences. While there was no difference in the disease duration between the two groups (both averaging around 2.4 years), the alpha synuclein seeding assay positive LRRK2-associated Parkinson’s cases were found to be:
- Younger (average 62 vs 69 years of age)
- With higher MDS-UPDRS part III (motor) scores (24 vs 17)
- More likely to be female.
There were also more cases of hyposmia in the positive cases (75% of the cohort vs just 18% of the negative group). Interestingly, preservation of the olfactory functions (a sense of smell) was associated with a negative alpha synuclein result across all of the genetic subgroups analysed.
The results of this study extends our knowledge about the potential utility of the alpha synuclein seeding test (which has been renamed the “SYNTap Test” for this particular version of it). In the conclusion of their report, the investigators noted that their “findings have immediate implications for clinical trial design, both to identify pathologically defined subgroups of people with Parkinson’s disease and to establish biomarker-defined at-risk cohorts“.
You mentioned that others have developed tests similar to the SYNTap Test. Have they published their results?
Yes they have. Here is one example:
 Title: Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies.
Title: Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies.
Authors: Poggiolini I, Gupta V, Lawton M, Lee S, El-Turabi A, Querejeta-Coma A, Trenkwalder C, Sixel-Döring F, Foubert-Samier A, Pavy-Le Traon A, Plazzi G, Biscarini F, Montplaisir J, Gagnon JF, Postuma RB, Antelmi E, Meissner WG, Mollenhauer B, Ben-Shlomo Y, Hu MT, Parkkinen L.
Journal: Brain. 2022 Apr 18;145(2):584-595.
PMID: 34894214 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers were validating the sensitivity and specificity of the alpha synuclein real-time quaking-induced conversion (or RT-QuIC) test on sample of cerebrospinal fluid from people with Parkinson’s. They collected samples from 74 people with Parkinson’s, 24 individuals with Multiple System Atrophy (MSA), 45 people with idiopathic REM sleep behaviour disorder (a prodromal risk factor for developing Parkinson’s) and 55 unaffected controls.
The RT-QuIC platform demonstrated 89% sensitivity and 96% specificity for Parkinson’s, but only 75% sensitivity for MSA and 64% for idiopathic REM sleep behaviour disorder.
The researchers concluded that “RT-QuIC adds value in diagnosing Parkinson’s“, and that “further analysis of longitudinally followed idiopathic REM sleep behaviour disorder patients is needed to better understand the relationship between α-synuclein RT-QuIC signature and the progression from prodromal to different synucleinopathies“.
Is this seeding assay test commercially available?
A company has been set up to develop the SYNTap Test – this is the alpha synuclein seeding assay that is mentioned in most of the papers listed above. This company is called Amprion (which should not be confused with another older Amprion company in Germany – they are different companies).
 Some of the researchers in the reports mentioned above are associated with this company. In the US, the SYNTap test was granted breakthrough device designation by the U.S. FDA in May 2019 (Source).
Some of the researchers in the reports mentioned above are associated with this company. In the US, the SYNTap test was granted breakthrough device designation by the U.S. FDA in May 2019 (Source).
What does breakthrough device designation mean?
The Breakthrough Devices Program is a voluntary program set up by the U.S. FDA in 2015 to expedite device access for life-threatening and debilitating diseases. The designation is granted to novel medical devices that have the potential to provide more effective treatment or diagnosis. It basically allows pharmaceutical and biotech companies to speed up the development process, and they receive extra guidance and support from the US FDA.
The designation must be requested by a company, but it does not mean that a particular test is approved for clinical use. The test must still meet the FDA’s rigorous standards for device safety and effectiveness in order to be authorized for marketing. While the SYNTap test is not yet FDA approved, physicians in the US can order the test if they want to test it on people exhibiting Parkinson’s symptoms (but it is currently not covered by insurance).
I do not want to have a lumbar puncture. Can the same results be achieved via a blood test?
This could be a completely separate post. The short answer here is that researchers are working on a blood test, but we are not there yet. Skin biopsies are also being explored (Click here to read an example of this research). Being so closely exposed to the brain, cerebrospinal fluid is currently considered to be the best source of sample material for the seeding assay test.
Can the tests be used to track progression of Parkinson’s?
For now, the available data indicates that the seeding assay approaches are best considered a binary test – a yes or no evaluation – rather than a measure of disease progression.
The research presented to date is mixed in terms of longitudinal or long-term data, with some preliminary results suggesting that alpha synuclein seeding may reduce with disease duration (Click here to read more about this).
Thus, at the moment, researchers are viewing the seeding assays simply as a potential component of a diagnostic process to aid clinicians. A lot more research is required to determine if it can detect Parkinson’s early in it’s development, and it will be interesting to compare it with other approaches attempting to achieve the same goals (Click here to read an SoPD post of another approach).
So what does it all mean?
There are efforts underway to try and redefine Parkinson’s based on biological markers. Alpha synuclein is an obvious place to start and has been the focus of much attention for very long time. What has resulted is a great deal of research indicating that alpha synuclein seeding assays of cerebrospinal fluid could be a very useful indicator of whether someone has a synucleinopathy (maybe one day it will be Parkinson’s specific, but I would like to see a bigger and better comparison with other synucleinopathies – like Dementia with Lewy bodies – before such claims are made).
I have to be honest here and say that I have never been a card carrying member of the ‘cult of synuclein’. I have always thought that if neurodegeneration was as simple as one protein misfolding, we would be too simple to understand it. And perhaps we are too simple to understand it, but I can recognise that the accuracy and sensitivity of the alpha synuclein seeding assay makes it a very interesting potential tool for the field. And the development of tools like this represent an important achievement, based on years of research by a lot of hard working people.
I am really intrigued by some of the nuances in the data presented thus far – such as why some of the LRRK2-PD samples are synuclein positive and others are not (does this overlap with postmortem pathological analyses?) – and one can see some immediate applications for these kinds of assays (clinical trials exploring alpha synuclein as a target should be assessing everyone being enrolled), but I have long worried about the emphasis that is placed on alpha synuclein in Parkinson’s research. I look forward to a day when a range of different biomarkers have been developed and validated which will hopefully provide us with a very clear idea of different types of Parkinson’s. But ideally by that point, we will have knocked this condition on the head (so to speak), and we won’t really need them. They will be important in getting us to that point though.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced and adapted from Foundationfar




Simon, you are a genius for informing us about the PD advances. Reading is a pleasure. Thank you for your amazing work. Please, keep it up!!!
LikeLike