|
# # # # The treatment of Parkinson’s has long been defined by medications that increase the level of a chemical called dopamine in the brain. The cells that produce dopamine are progressively lost in this condition. To date, dopamine-replacement therapies have largely been based on orally administered pills that result in fluctuating levels of dopamine across the day. But now regulators have approved a new treatment that provides continuous levels of dopamine. It is called Produodopa. In today’s post, we will look at what is dopamine, why the treatments supplementing dopamine fluctuate across the day, and how Produodopa may be able to help with this. # # # # |
 Source: Bwcharity
Source: Bwcharity
One neurologist described it to me as the ‘Deep brain stimulation killer’.
I’m not so sure about that.
Others have suggested that it has the potential to be a revolutionary shift for the future treating Parkinson’s.
Again, I wouldn’t go that far, but it could be a very important step forward in better management of motor symptoms in advanced Parkinson’s.
What are we talking about here?
It is called Produodopa, and it is a new system of continuous delivery of levodopa that has been developed by the pharmaceutical company AbbVie.
Produodopa was launched in the European Union in January 2024 (Click here to read more about this), and was approved for clinical use in England by the National Institute for Health and Care Excellence (NICE) on the October 26th 2023 (Click here to read more). It is still awaiting approval in the USA.
And what exactly is Produodopa?
Produodopa is a subcutaneous 24-hour delivery system for foslevodopa-foscarbidopa.
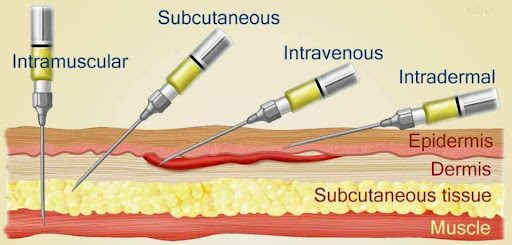
Ok. Lots of questions here. First, what does subcutaneous mean?
Subcutaneous comes from the Latin words ‘sub’, which means “under”, and ‘cutis’, meaning “skin”. In the context of Produodopa, it refers to a short needle being used to inject two drugs into the tissue layer under the skin, where they can be slowly absorbed into the body.
 Source: Shagbarkridge
Source: Shagbarkridge
Got it. Next, what is the drug? Foslevodopa-foscarbidopa?
Not a drug, but drugs. Pural. Produodopa is a combination of two drugs.
The first is Foslevodopa, which is a prodrug (a prodrug is an inactive molecule that is metabolized by the body into an active drug).
Foslevodopa acts as a prodrug for levodopa.
Levodopa is the main ingredient for the production of dopamine.
What is dopamine?
Dopamine is a chemical is the brain that plays a role in many basic functions of the brain, such as motor co-ordination, reward, and memory.
It works as a signaling molecule – a way for brain cells to communicate with each other. Dopamine is released from neuron, and it binds to target neurons, initiating biological process within those cells.
In this manner, it is called a neurotransmitter.
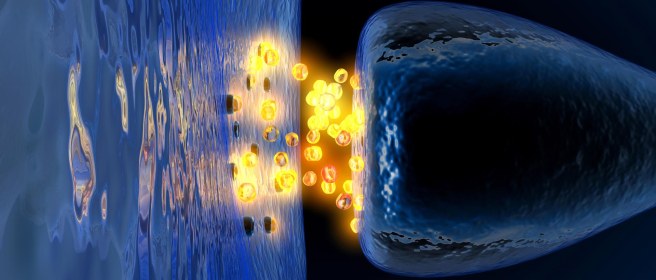 Dopamine being released by one cell and binding to another. Source: Truelibido
Dopamine being released by one cell and binding to another. Source: Truelibido
The dopamine neurons in the substantia nigra region of the brain generate the bulk of the dopamine in your brain, but they release most of it in different areas of the brain. The primary regions of that release are areas of the brain called the putamen and the caudate nucleus. The dopamine neurons of the substantia nigra have long projecting branches (or axons – the black curving lines in the image below) that extend a long way up into the brain to the putamen and caudate nucleus, so that dopamine can be released there.
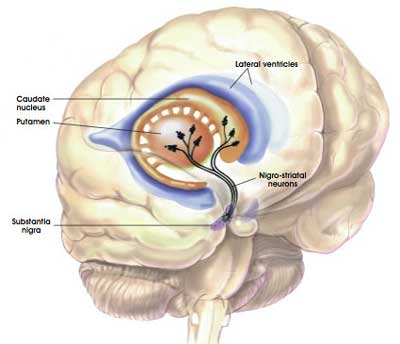
The projections of the substantia nigra dopamine neurons. Source: MyBrainNotes
Ok, so why is Foslevodopa – the prodrug for levodopa, which is needed in the production of dopamine – important for Parkinson’s?
Because in Parkinson’s, the dopamine neuron ‘axon’ extensions that project to the putamen and caudate nucleus gradually disappear as the dopamine neurons of the substantia nigra are lost. We are not sure why, but Parkinson’s is defined by this loss of dopamine neurons and reduction in levels of dopamine in the brain.
By the time a person is presenting the motor features characteristic of Parkinson’s, and being referred to a neurologist for diagnosis, they have already lost approximately 50% of the dopamine producing neurons in the substantia nigra.
 The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
This results in a reduction of dopamine being released in the putamen and caudate, which leaves the movement areas of the brain gradually becoming more inhibited. And this increase in inhibition shows itself clinically by the slowness of and problems initiating movement.
One of the primary treatments for Parkinson’s is levodopa. As discussed above, it is an ingredient in the production of dopamine.
How does levodopa work?
When you take an levodopa tablet, the pill will be broken down in your stomach and the levodopa will enter your blood through the intestinal wall. Via your bloodstream, it arrives in the brain where it will be absorbed by cells. Inside the cells, another chemical (called DOPA decarboxylase) then changes it into dopamine. That newly produced dopamine helps to replace the missing dopamine in the brain and alleviate the motor features of Parkinson’s.
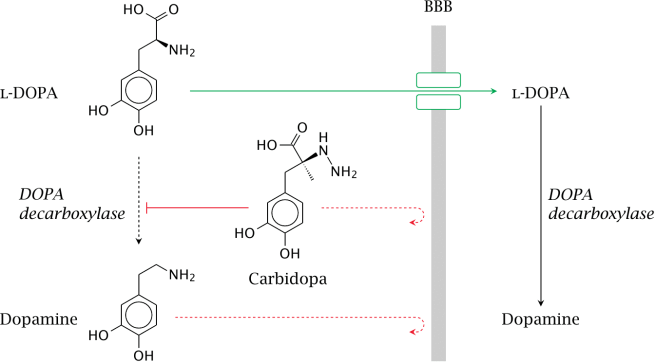
The production of dopamine, using levodopa. Source: Watcut
Ok, so what is the foscarbidopa part of the foslevodopa-foscarbidopa combination? What exactly is foscarbidopa?
Outside the brain, there is a lot of DOPA decarboxylase in other organs of the body, and if the activity of this enzyme is not blocked then the effect of levodopa is reduced in the brain.
Why?
Because the levodopa absorbed from the gut will be turned into dopamine in the blood system before it reaches the brain.
To this end, people with Parkinson’s are also given another drug with their levodopa treatment. This second drug is called carbidopa.
Carbidopa inhibits DOPA decarboxylase outside of the brain (Carbidopa does not cross the blood-brain-barrier, or the BBB in the image above which is a protective membrane covering the entire brain).
And foscarbidopa is a prodrug for carbidopa?
Exactly.
Foscarbidopa is inactive until it is in the body and metabolised into carbidopa.
Ok, but why don’t we just use dopamine to treat Parkinson’s?
We can not use dopamine directly because:
- It can not cross the blood brain barrier (described above), and
- Dopamine is broken down very quickly in the body. There are lots of enzymes floating around that can digest dopamine so that it doesn’t do anything it’s not supposed to.
I see. So why is this new Produodopa treatment special? Why is it even needed? How is it different to normal Parkinson’s treatments?
Because Produodopa offers a 24-hour delivery of foslevodopa-foscarbidopa, meaning that the body is getting a steady level of dopamine supply.
To understanding that sentence, it is important to appreciate the current situation with levodopa treatment. At present, when a levodopa tablet is consumed and levodopa enters the brain, there is a rapid increase in the levels of dopamine. This results in a ‘spike’ in the supply of dopamine that will last for the next few hours, before the dopamine is eventually used up.
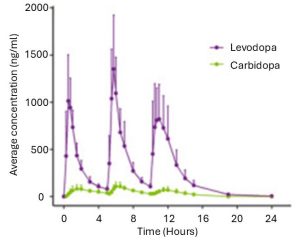 Source: PMC
Source: PMC
As the effects of the levodopa tablet wear off, another tablet will be required. This use of multiple levodopa pills across the day gives rise to a wave-like shape to the dopamine levels in the brain over the course of the day (see the figure below). The first pill in the morning will quickly lift the levels of dopamine enough that the individual will no longer feel akinetic (unable to move). This will allow them to be able to function with normal controlled movement for several hours (this is referred to as ON time) before the levodopa begins to wear off. As the levodopa wears off, the dopamine levels in the brain drop back towards levels that will leave the person feeling akinetic and at this point another levodopa tablet is required.

A hypothetical illustration of dopamine levels over a day
After several years of levodopa use, many people with Parkinson’s will experience a weaker response to each tablet. They will also find that they have more time during which they will be unable to move (exhibiting akinesia). This is simply the result of the slow progression of Parkinson’s – levodopa treats the motor features of the condition but only hides/masks the fact that the disease is still progressing.
To combat this shorter response time, the dose of levodopa is usually increased. This will result in increasing levels of dopamine in the brain (as illustrated by the higher wave form over time in the image below). Gradually it will take more levodopa medication-induced dopamine to lift the individual out of the akinetic state.
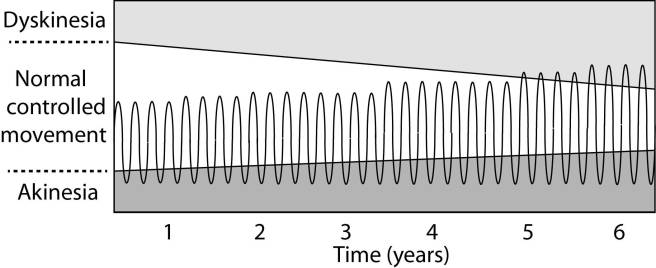
Again this illustration is hypothetical (situation differs between individuals)
This increasing of levodopa dosage, however, results in too much dopamine being present in the brain at times. And this situation is often associated with the gradual development of abnormal involuntary movements that appear when the levels of levodopa induced dopamine are the highest.
These are the involuntary muscle movements that we refer to as dyskinesias.
What are dyskinesias?
Dyskinesias (from Greek: dys – abnormal; and kinēsis – motion, movement) are a category of movement disorders that are characterised by involuntary muscle movements. And they are certainly not specific to Parkinson’s.
But in the case of Parkinson’s, dyskinesias have generally been believed to be associated with long-term use of levodopa.
NOTE: Long-term use of levodopa is not a certainty for developing dyskinesias, but there is an association. It will differ from person to person.
A more continuous supply of levodopa (like that provided by Produodopa) would hopefully do away with the wave-like levels – the over-supply and under-supply – of dopamine in the brain, and allow for the better management of motor symptoms (reducing the risk of developing dyskinesias).
I see. So what research has been conducted on Produodopa?
Well, it is important to understand that Produodopa is a new development on an older product called Duodopa.
Duodopa is a delivery system that injects a gel-based formulation of levodopa/carbidopa into the small intestine, where it can be absorbed into the blood stream:
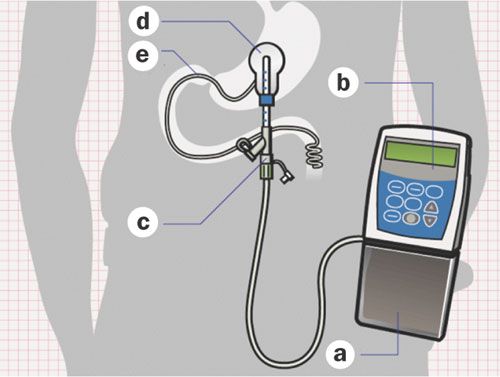 Duodopa. Source: Nature
Duodopa. Source: Nature
This approach has been approved for clinical use in the European Union since 2004, and in the U.S. since 2015. The main draw-back of duodopa, however, is that it requires a surgical procedure and the implantation of tubing into the intestines (see figure above).
These details with Duodopa lead to the development of ABBV-951 (which would go on to be called Produodopa). It does not require any surgical procedure and the foslevodopa-foscarbidopa combination is delivered via a needle under the skin. And there is a large patch keeping the needle covered and in place over the day:
 Source: Medicines
Source: Medicines
The whole Produodopa system can sit on one’s belt around the hips:
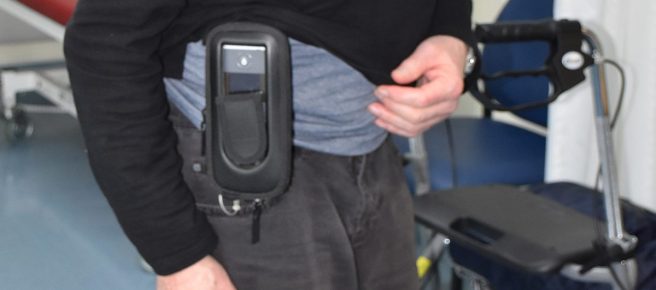 Source: Movementdisordersclinic
Source: Movementdisordersclinic
A bit chunky, but the continuous flow of Foslevodopa/Foscarbidopa means a stable supply of dopamine.
The idea is no more pills and hopefully less OFF time.
What is OFF time?
“OFF” time (also known as “wearing off”) occurs between doses of medication when the Parkinson’s symptoms return. “ON” time is the period when the medication is working and the symptoms are under control, allowing the individual to move more freely.
OK. So what research has been conducted on Produodopa?
The research on Produodopa has been extensive and a lot of it has already been published.
It began with this report:
 Title: Foslevodopa/Foscarbidopa: A New Subcutaneous Treatment for Parkinson’s Disease.
Title: Foslevodopa/Foscarbidopa: A New Subcutaneous Treatment for Parkinson’s Disease.
Authors: Rosebraugh M, Voight EA, Moussa EM, Jameel F, Lou X, Zhang GGZ, Mayer PT, Stolarik D, Carr RA, Enright BP, Liu W, Facheris MF, Kym PR.
Journal: Ann Neurol. 2021 Jul;90(1):52-61.
PMID: 33772855 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers described some of the first investigations of the foslevodopa/foscarbidopa combination in humans. Specifically, they explored different ratios of the two agents (ranging from 4:1 to 20:1) and infused them into healthy volunteers for up to 72 hours. Across the dosing ratios, the investigators found that the 20:1 (foslevodopa‐to‐foscarbidopa) ratio was the most appropriate for stable delivery of dopamine:
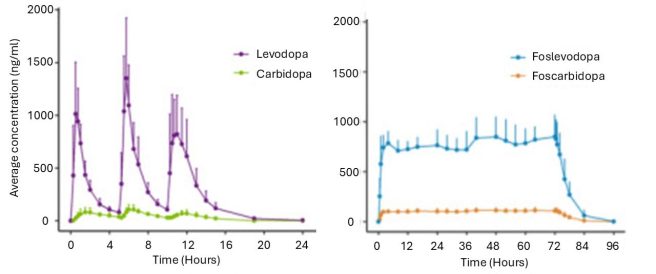 Normal oral levodopa treatment VS ProduodopaSource: PMC
Normal oral levodopa treatment VS ProduodopaSource: PMC
In the images above, you will see the current oral levodopa pill-based delivery of levodopa (in combination with carbidopa) across a 24 hour period in the left hand side graph. The levodopa levels in blood over time are presented in the purple line. Note the spiking nature of the levodopa levels across the graph, occurring three times as three oral pills are taken at 0, 4 and 9 hours across the 24 hour period (no pill was taken during the period of sleep). And now compare that with the right hand graph, where you will see an elevation and then stable delivery of foslevodopa (the blue line) over the entirety of a 72 hour period (this was the output from the 20:1 foslevodopa‐to‐foscarbidopa ratio).
This positive initial outcome was quickly followed by testing of the Produopdopa system in a Parkinson’s cohort over a 72 hour period.
The results of that study are published in this report:
 Title: Foslevodopa/Foscarbidopa Is Well Tolerated and Maintains Stable Levodopa and Carbidopa Exposure Following Subcutaneous Infusion.
Title: Foslevodopa/Foscarbidopa Is Well Tolerated and Maintains Stable Levodopa and Carbidopa Exposure Following Subcutaneous Infusion.
Authors: Rosebraugh M, Liu W, Neenan M, Facheris MF.
Journal: J Parkinsons Dis. 2021;11(4):1695-1702.
PMID: 34366380 (This report is OPEN ACCESS if you would like to read it)
This was a Phase 1, single-blind (meaning that the investigators were blind to the treatment being tested) study. It was conducted in 28 people with Parkinson’s who were treated for 72 hours with Produodopa. When comparing the spiking levels of oral levodopa treatment (modelled), the Produodopa approach provided very stable delivery of levodopa for the 72 hour period across the participants:
 Source: PMC
Source: PMC
These studies (and others) were followed by a 12 week Phase 3, double-blind study of the foslevodopa/foscarbidopa treatment (versus oral immediate-release LD/CD) in 141 people with Parkinson’s.
The results of that study were published in the Lancet Neurology journal in late 2022:
 Title: Safety and efficacy of continuous subcutaneous foslevodopa-foscarbidopa in patients with advanced Parkinson’s disease: a randomised, double-blind, active-controlled, phase 3 trial.
Title: Safety and efficacy of continuous subcutaneous foslevodopa-foscarbidopa in patients with advanced Parkinson’s disease: a randomised, double-blind, active-controlled, phase 3 trial.
Authors: Soileau MJ, Aldred J, Budur K, Fisseha N, Fung VS, Jeong A, Kimber TE, Klos K, Litvan I, O’Neill D, Robieson WZ, Spindler MA, Standaert DG, Talapala S, Vaou EO, Zheng H, Facheris MF, Hauser RA.
Journal: Lancet Neurol. 2022 Dec;21(12):1099-1109.
PMID: 36402160
In this study, the investigators reported a significantly greater improvements in motor fluctuations in the Produodopa group compared with oral levodopa-carbidopa treatment over the 12 week period. The most frequent adverse events in the Produodopa group were issues at the infusion site, where the needle interacts with the skin. Most of these were considered non-serious and mild-moderate in their severity, but they did lead to premature discontinuation from the study of 16 (22%) of the 74 participants in the Produodopa group (this was compared to just one of the 67 participants in the oral levodopa-carbidopa group).
A follow up 12-month open label study was conducted to assess the longer term safety, tolerability and efficacy of the 24-hour/day Produodopa treatment approach.
The results of that study were published in this report:
 Title: Continuous Subcutaneous Foslevodopa/Foscarbidopa in Parkinson’s Disease: Safety and Efficacy Results From a 12-Month, Single-Arm, Open-Label, Phase 3 Study.
Title: Continuous Subcutaneous Foslevodopa/Foscarbidopa in Parkinson’s Disease: Safety and Efficacy Results From a 12-Month, Single-Arm, Open-Label, Phase 3 Study.
Authors: Aldred J, Freire-Alvarez E, Amelin AV, Antonini A, Bergmans B, Bergquist F, Bouchard M, Budur K, Carroll C, Chaudhuri KR, Criswell SR, Danielsen EH, Gandor F, Jia J, Kimber TE, Mochizuki H, Robieson WZ, Spiegel AM, Standaert DG, Talapala S, Facheris MF, Fung VSC.
Journal: Neurol Ther. 2023 Dec;12(6):1937-1958.
PMID: 37632656 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers found that after 52 weeks of treatment there was a 59% average reduction in “OFF” time and a 41% increase in “ON” time without troublesome dyskinesia compared to baseline:
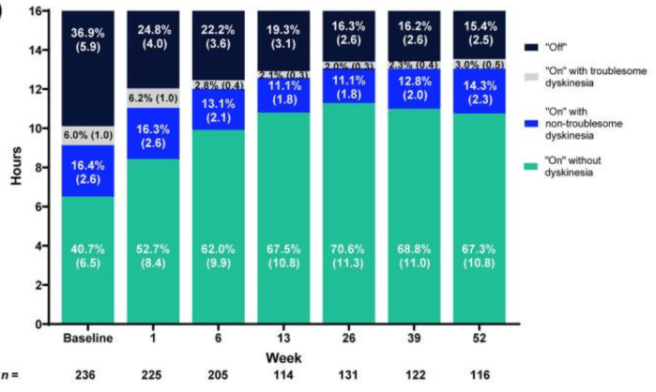 Source: PMC
Source: PMC
They also reported that the percentage of patients experiencing morning akinesia dropped from 77.7% at baseline to 27.8% at week 52. And, in addition to that, participants reported improvements in sleep quality and quality of life measures.
Very interesting. Has Produodopa been approved for clinical use? Is it available?
As discussed near the top of this post, Produodopa was launched in the European Union in January (Click here to read more about this), and was approved for clinical use in England by the National Institute for Health and Care Excellence (NICE) on the October 26th 2023 (Click here to read more).
It is still awaiting approval by the FDA in the USA.
AbbVie and the regulators are being very cautious about the roll out in the UK, with only 1000 units being made initially available. You will need to talk with you clinician to determine if you are eligible.
But there has been considerable interest already, partly due to some media attention, with videos like this being shared:
Looks encouraging. Is AbbVie the only biotech that has developed this type of delivery system for levodopa?
No, it is not.
Another biotech company called Neuroderm currently has a very similar product – called ND0612 – under development.
In January, NeuroDerm announced positive results for their 12 week Phase 3 “BouNDless ” clinical trial of ND0612 in people with Parkinson’s (Click here to read more about this). They reported that ND0612 “showed superior efficacy compared to oral LD/CD for the primary endpoint (“ON” time without troublesome dyskinesia, also called “Good ON” time) and the key secondary endpoint (“OFF” time)”
Interestingly, only 6.3% of patients randomised to the ND0612 treatment group discontinued during the trial.
So what does it all mean?
This post is not an advert for AbbVie or Produodopa. I have had no communication with the company (nor any of the competing companies).
I have written this post simply because I genuinely think this is a really interesting development in the treatment of Parkinson’s symptoms. Firstly, the stable delivery of levodopa will hopefully reduce many of the troublesome side effects associated with intermittent oral levodopa delivery. The Produodopa product is currently being oriented towards more advanced cases of Parkinson’s in the roll out, but further down the road it will be interesting to see if this kind of treatment approach can make the management of early motor symptoms more agreeable for more recently diagnosed individuals.
Second, these stable delivery systems allow for more personalised dosing based on individual needs, across the day (morning, day and night). Personalisation is currently done with oral levodopa treatment (in terms of dose), but the kind of fine tuning that will come with products like Produodopa will hopefully be a blessing for the Parkinson’s community.
It is pleasing to see that the regulators and industry players are being careful with the real world roll out of the product. There will be a wealth of knowledge to be collected from the broader use of this technology. And that will help with future iterations in the evolution of it. One key detail that is missing at present is a feedback loop (in which the level of levodopa in the blood stream is measured and that information can be used to determine the amount of levodopa is required). But this kind of adaptive approach is being explored (Click here and here to read more about this).
Like I said at the top of this post, I’m not sure that it will be the deep brain stimulation killer or a revolution in the treatment of Parkinson’s, but it certainly is an encouraging step forward.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
ADDENDUM – 17th October, 2024
The U.S. Food and Drug Administration (FDA) has finally approved VYALEV™ (previously known as Produodopa or foscarbidopa and foslevodopa) as “the first and only subcutaneous 24-hour infusion of levodopa-based therapy for the treatment of motor fluctuations in adults with advanced Parkinson’s” (Click here to read more about this).
EDITOR’S NOTE: The author of this post is an employee of Cure Parkinson’s, so he might be a little bit biased in his views on research and clinical trials supported by the trust. That said, the trust has not requested the production of this post, and the author is sharing it simply because it may be of interest to the Parkinson’s community.
The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
In addition, many of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was sourced from abbviepro


It is not widely known that l-DOPA is a substrate for gut and liver Cyp3A4. There is a ‘grapefruit-juice ‘ effect that can double the on-time of the oral drug. However there are no data AFAIK re plasma levels. Chronic suppression of Cyp3A4, the major xenobiotic- metabolising gut/liver cytochrome, may not be desirable. Cutaneous delivery avoids cyp3A4 and that may well be beneficial.
LikeLiked by 2 people
Vyalev – Medicare may cover it around July of 2025, I was told by my doctor – till then commercial / employer provided insurance only. And even that may take a while to roll out – supply and demand I guess can be bumpy.
This seems to be a real help with people who have slow stomach emptying or gastroparesis. Is there a percentage you think of pwp who have this condition? I have seen anywhere from 12% to 100%.
Have you seen the news about a-dopamine – pure dopamine piped directly into the brain? https://www.medscape.com/viewarticle/direct-brain-dopamine-infusion-promising-parkinsons-disease-2024a1000hzj
LikeLike