|
# # # # At the end of each year, it is a useful process to take stock and review what we have learnt over the last 12 months. 2025 has been a very busy year for Parkinson’s research, with a lot of clinical trial results being reported and new insights being made. In today’s post, we will consider three big Parkinson’s-related research takeaways of 2025 (based on our humble opinions here at the SoPD), and then we will provide an extended overview of some of the important pieces of news from the last 12 months (Be warned: this is a rather long post). # # # # |
 Source: NYTimes
Source: NYTimes
This is little unit is KJ Muldoon.
Research-wise, 2025 was a pretty big year for him (in fact, he was one of Nature’s 10 people who shaped science in 2025).
Born on the 1st August, 2024, in Clifton Heights, Pennsylvania, KJ was the fourth child of Kyle and Nicole Muldoon. The day after he was born it was noted that he was unusually sleepy and averse to feeding. Blood work quickly showed that he was accumulating ammonia in his blood, and a genetic test revealed that he had a mutation that caused carbamoyl phosphate synthetase I (CPS1) deficiency.
KJ was too young for a liver transplant which was the only major treatment available at the time, so his treatment options were looking extremely limited.
What happened next was part of what made 2025 an amazing year:
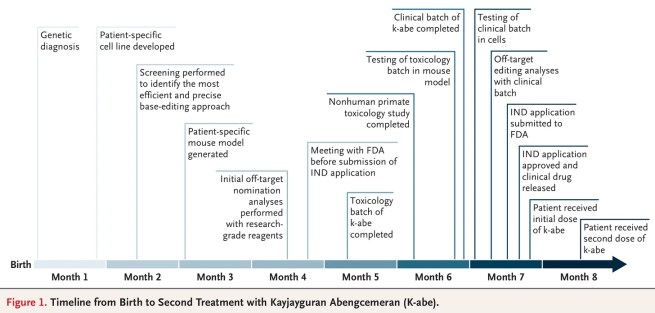
The kid doesn’t know it yet, but he made medical history as the first human to receive a personalized CRISPR-based gene therapy treatment (Click here to read the research report behind this story). And here is a timeline of events in his treatment story:
And very recently, KJ took his first steps (Click here to see this).
Forget about all of the idiotic nonsense flooding social media and all of the witless utterances coming from our so called “leaders” and all of the talking heads on normal media.
Stories like KJ’s are made 2025 an incredible year. Part of humanity truly striving for a better future.
And this was only one story among a huge bag of uncelebrated scientific advances that occurred this year. Advances such as:
- A team of researchers at Roche and Boston’s Children’s Hospital set a new record for the fastest human genome sequencing and analysis. It took them just under 4 hours to sequence the whole genome – in the 2010s it took 3 days (Click here to read more about this).
- The second chikungunya vaccine (‘Vimkunya’) was approved. Chikungunya is a disease spread by mosquitoes (similar to dengue), which can cause months of joint pain and in some rare cases, paralysis. Vaccines do work (Click here to read more about this).
- The seemingly unstoppable growth of renewable energy (Click here to read more about this).
- The FDA gave a green-light to the first multi-person clinical trials of pig kidney transplants, which will hopefully help ease the global shortage of donor organs (Click here to read more about this).
- The Vera C. Rubin Observatory came online.
- The FDA has approved 44 brand new drugs – more than twice as many as were approved in 2010.
- Lenacapavir is a long-acting antiviral injection for HIV received approval in the US for HIV prevention (Click here to read more about this).
- The UK became the first country to offer a vaccine against gonorrhea (Click here to read more about this).
- Researcher combined AI models RFDiffusion and AlphaFold2 to create a ‘multi-step enzyme’ for the first time, and that enzyme has never been seen before in nature (Click here to read more about this and click here to read an exemplar).
- In October, tech company Google announced that its “quantum echoes” algorithm proved 13,000 times faster than a classical computer at predicting molecular structures (Click here to read more about this).
- Mitochondria were discovered to be critical for memory formation in immune T cells and have an unexpected role in tissue healing (Click here and here to read more about this, respectively).
Below is a list of some of the more interesting Parkinson’s research findings of the year – by month, but starting with the top three according to the team here at SoPD HQ.
|
# EDITOR’S NOTE: The author of this blog is the director of research at the medical research charity Cure Parkinson’s. For the purpose of transparency and to eliminate any sense of bias, where Cure Parkinson’s is a funder of the research it shall be noted. The selection of research topics below are based on his opinion alone and do not reflect the thoughts of any other parties. # |
The 3 main SOPD highlights in Parkinson’s-related research for 2025
(in no particular order – just our opinion)
1. The largest clinical trial for Parkinson’s (ever) in the UK began:
After decades of testing individual drugs in purpose built/stand alone clinical trials, the world of Parkinson’s research has finally embraced large scale, adaptive platform trial designs. These new projects will act as continuous conveyor belts for testing new therapies. By regularly analysing the results, the investigators will be able to determine if a particular agent on their platform is having an impact on the disease and if not, they will be able to replace it with another alternative agent.
In 2025, the first of these platforms, the Edmond J. Safra Accelerating Clinical Trials for Parkinson’s Disease (EJS ACT-PD) multi-arm, multi-stage clinical trial platform for Parkinson’s started recruiting. This has been a MASSIVE project for the Parkinson’s community, with dedicated working groups of volunteers from all walks of life getting involved. The trial team are looking for 1600 participants, who will be randomised across 4 arms (3 different drugs & a placebo). If you live in the UK (there are 40 sites across England, Scotland, Wales and Northern Ireland), register your interest here. (Cure Parkinson’s is a funder of this project)
For readers outside of the UK, fear not. There are additional multi-arm clinical trial platforms being set up. For example, there are:
- the MASTER trial in France
- the HYDRA platform in Norway
- the SLEIPNIR platform in Norway (Cure Parkinson’s is a funder of this project)
- the Path to Prevention Platform trial in the US
- the ongoing Australian Parkinson’s Mission trials (the first of these trials will be reporting in 2026)
An international consortium of these platforms – the Global Alliance for Parkinson’s Platforms (or GAPP) – has also been set up, and you will learn more about this in 2026.
2. Nation-wide study highlights drug repurposing candidates:
A big part of my job at Cure Parkinson’s is identifying drugs or interventions that have the potential to slow, stop or reverse Parkinson’s. As such, one of the more interesting research reports I read in 2025 was a nationwide observational cohort study (2004–2020) that involved using data from Norwegian health registries (4.5 million individuals). By analysing the case histories of people who passed away with Parkinson’s (N=14,289 individuals), the researchers were able to identify 23 drugs associated with reduced mortality risk in Parkinson’s at 8 years. The authors wrote “Our study identified several drugs with potential disease-modifying properties that could be candidates for future clinical trials. It highlights the potential of repurposing existing medications to advance drug development” (Click here to read more about this and click here to read an editorial about the results).
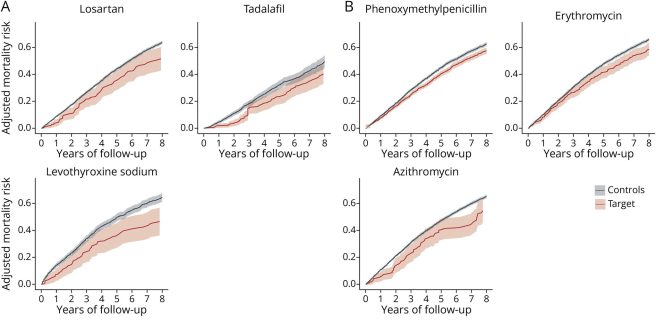 Source: Neurology
Source: Neurology
Some of the agents highlighted in this study can’t be taken forward for clinical testing (for various reasons), but there is certainly a lot of food for thought and it will be interesting to see whether this data can be replicated using another large dataset.
3. Could some LRRK2 variants initially be protective?:
For a long time, LRRK2 genetic variants have been associated with increasing one’s risk of developing Parkinson’s, but perhaps this binary view of the world is a little simplistic. New research in 2025 reported that iPSC-derived neurons carrying LRRK2 variants upregulate the secretion of extracellular vesicles; Unbiased proteomics found that autophagic cargos were enriched. In other words, the cells with LRRK2 variants were spitting out more rubbish than cells without these variants – and this could be some kind of compensating mechanism.
The investigators proposed “that this increased secretion contributes to the maintenance of cellular homeostasis, delaying neurodegenerative disease progression over the short term while potentially contributing to neuroinflammation over the longer term”. These results are preliminary and more research is required to determine if the finding is relevant to what is actually happening in the human brain, but the data is intriguing and may have important implications for how we interpret the data coming from clinical trials investigating LRRK2 inhibition in Parkinson’s – the first of which will be reporting results in late 2026 (Click here to read more about this and click here to read an opinion piece on this research).
# # # # # # #
The three pieces of research news above were what grabbed my attention the most in 2025, but it was a very full year of new data and findings. Below, we’ll go month-by-month and discuss some of the other highlights.
Let’s begin with:
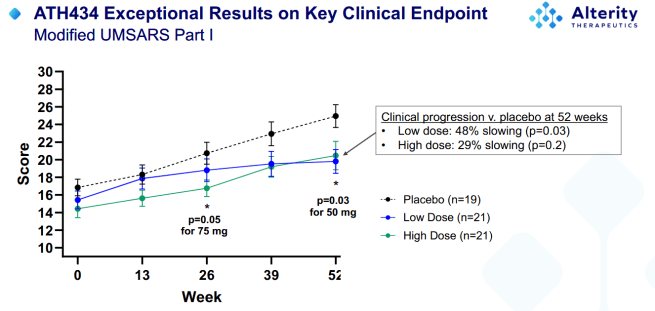
1. Encouraging Phase 2 results from Alterity Therapeutics:
ATH 434 (formerly PBT 434) is an orally bioavailable, small molecule, inhibitor of α-synuclein aggregation, being developed by Alterity Therapeutics. In January, the company announced encouraging topline results from their 12 month-long “ATH434-201” randomized, double-blind, placebo-controlled Phase 2 clinical trial in 77 patients with early-stage multiple system atrophy. This study found that ATH434 was safe and well tolerated, reduced iron accumulation, and provided a 48% slowing of clinical progression (Click here to read the press release and click here to see the results presentation).
2. New data suggesting Deep brain stimulation could be disease modifying:
Using Parkinson’s Progression Markers Initiative (PPMI) data, researchers found that circulating lymphocytes progressively decrease in Parkinson’s & can predict future motor symptom progression. Interestingly, they also found that deep brain stimulation causes a shift from the pro-inflam CD4+ Th17 cells to anti-inflam CD4+ Treg cells (Click here to read more about this).
3. Tetanus–diphtheria vaccinations:
Using national health providers data in Israel, a retrospective case–control study finds tetanus–diphtheria vaccination significantly reduced the occurrence of Parkinson’s; Adjusted OR of 0.17 (95% CI [0.04, 0.70]) for PD Dx within 5 years post-vax (Click here to read more about this).
1. The “Exenatide-3” Phase 3 clinical trial results were published:
The results from the “Exenatide-3” Phase 3 clinical trial of the diabetes drug Bydureon in 194 people with Parkinson’s (2yrs treatment) have been published; Safe & well tolerated, but “no evidence to support exenatide as a disease-modifying treatment” (Click here to read more about this and click here to read a summary of the results). For those interested in learning more about the Phase 3 exenatide results, Prof Tom Foltynie gave the following presentation at the Cure Parkinson’s Autumn Research Update meeting last November (Cure Parkinson’s was a funder of substudies in this trials):
2. The FDA approves adaptive Deep Brain Stimulation:
Medtronic announces US FDA approval of their BrainSense™ Adaptive deep brain stimulation (aDBS) & BrainSense™ Electrode Identifier. By recording brain activity and allowing stimulation patterns to be adjusted to the needs of the brain, this represents a more personalised deep brain stimulation for Parkinson’s (Click here to read more about this).
3. ASKBio receives support for gene therapy AAV-GDNF:
ASKBio (subsidiary of Bayer) receives US FDA Regenerative Medicine Advanced Therapy (RMAT) designation for their investigational gene therapy AAV-GDNF targeted at Parkinson’s. RMAT provides recipients with enhanced access to the FDA and is designed to speed up the regulatory process (Click here to read more about this).
1. Drug repurposing:
A systematic study of the UK Biobank finds genetically supported targets & drug repurposing for brain aging; 38k individuals involved (81 with Parkinson’s); Higher levels of MAPT are positively associated with elevated glucose levels & increased risk of PD. 29 drugs highlighted; 13 (cholecalciferol, dasatinib, diclofenac, doconexent, estradiol, hydrocortisone, mecamylamine, nicotine, prasterone, quercetin, resveratrol, sirolimus, & testosterone) associated with clinical trials for aging-related indications (Click here to read more about this).
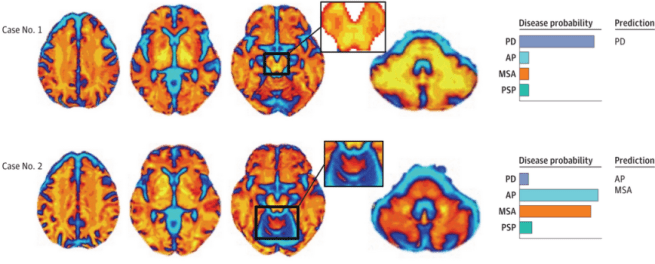
2. Automated Imaging Differentiation for Parkinsonism
A new prospective multicenter cohort study of Automated Imaging Differentiation for Parkinsonism suggests using this approach in the diagnostic workup for common parkinsonian syndromes, such as Parkinson’s & atypical parkinsonism (Click here to read more about this and click here to read a press summary on this research).
3. Long-term air pollution exposure & genetic susceptibility
A gene-environment interaction study, a combination of long-term air pollution exposure & genetic susceptibility “strongly contributed to the risk of developing Parkinson’s”; A meta-analytical assessment of studies conducted in central California & Denmark (Click here to read more about this).
1. The Bluerockers report Phase 1 results:
Bluerock Therapeutics (an independent subsidy of Bayer) published the 18 month data from their Phase 1 study of bemdaneprocel (cryopreserved, off-the-shelf dopaminergic neuron progenitor cell product) for Parkinson’s; 18F-FDOPA uptake increase indicates good graft survival (Click here to read more about this).
2. News from the Kyoto cell transplantation trial:
In addition, the Kyoto team have published their Phase 1/2 data as well! 24 months of data from their clinical trial of transplantation of dopaminergic progenitors derived from iPS cells in 7 people with Parkinson’s; Results also look good! (Click here to read more about this, click here for an editorial on the study, and click here for a news summary).
3. Forget B1. Be one with B6:
New research finds dysregulation in the kynurenine pathway in Parkinson’s is linked to peripheral and CNS inflammation and low vitamin B6 status. The investigators suggest Parkinson’s is characterized by vitamin B6-dependent inflammatory kynurenine pathway dysfunction. Using Mass-spec of blood & CSF metabolites from 158 controls & 177 PD cases, the researchers also identify patient subgroups with distinct kynurenine pathway profiles that displayed certain PD clinical features (Click here to read more about this).
 1. The results of the Transeuro clinical trial have been published.
1. The results of the Transeuro clinical trial have been published.
This was a cell transplantation study (NCT01898390) involving 11 individuals with Parkinson’s transplanted. The results indicate improvements in brain imaging (FDopa PET) at 18 months post transplantation in 7 participants (Click here to read more about this). (Cure Parkinson’s was a funder of this project)
2. New data from the GDNF gene therapy trial:
Fresh 18 month data from the phase 1b single-arm, open-label clinical trial of AAV-GDNF in Parkinson’s by ASK bio (Bayer) has been published; N=11; Well tolerated & associated with numerical stability (mild cohort; <5 yrs since dx) & improvement (moderate cohort – click here to read more about this).
3. We are what we eat:
New research finds long-term consumption of ultraprocessed foods (UPF) positively associated with nonmotor prodromal Parkinson’s features, constipation, body pain, & depressive symptoms); Could lowering UPF consumption be preventative? (Click here to read more about this, click here for an editorial on this research, and click here to read a press summary).
1. Another inflammasome drug gives encouraging results:
Ventyx Biosciences announces positive top-line data from their Phase 2a, 28-day, open label safety & biomarker study evaluating the NLRP3 inhibitor VTX3232 in 10 individuals with early-stage Parkinson’s; NCT06556173; Good pharmacokinetics – the agent was safe & tolerable (Click here to read more about this).
2. More data on tanganil (acetyl-leucine):
A 2-year case study in a patient with Parkinson’s & REM sleep behaviour disorder (RBD); Treatment helps with RBD symptoms; PD analysis is in the supplemental data file (Click here to read more about this).
3. Digital biomarker results supporting PASADENA:
Exploratory digital outcome measures of motor sign progression in Parkinson’s collected by Roche in their Phase 2 PASADENA study of the anti-alpha-synuclein monoclonal antibody prasinezumab have been published – encouraging data for digital biomarkers (Click here to read more about this and click here to read a press summary on this).
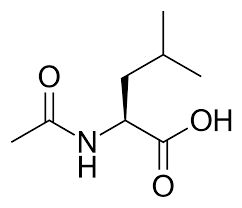
1. The Canadian Ambroxol study results:
The results of the ambroxol in Parkinson’s dementia study have been published: This was a 52-week, Phase 2, double-blind, placebo-controlled, randomized clinical trial involving 55 participants, randomised to low dose (525mg), high dose (1050mg) or placebo. It was a small study, and the main takeaways were that the treatment was safe & well tolerated in 55 people with Parkinson’s dementia. Target engagement was also demonstrated in that ambroxol elevated β-glucocerebrosidase (GCase) levels in the cerebrospinal fluid. The investigators reported no difference in cognitive scores, but neuropsychiatric symptoms got worse in the placebo group! (Click here to read more about this and click here to read a press summary about the results).
2. APOE ε4 is a pleiotropic immune modulator:
Researchers looked at proteomics data from 11,270 individuals & find a conserved APOE ε4-associated pro-inflammatory immune signature persistent across the brain, CSF & plasma irrespective of neurodegenerative conditions; Results highlights “a fundamental, disease-independent biological vulnerability to neurodegeneration. This work reframes APOE ε4 as a pleiotropic immune modulator rather than an Alzheimer’s-specific risk gene, providing a foundation for…early intervention strategies across neurodegeneration” (Click here to read more about this and click here to read an editorial on this research).
3. Combination therapies – All the cool kids are doing them:
Researchers conducted human transcriptomic & drug repurposing analyses to identify letrozole + irinotecan combo for Alzheimer’s. The combo therapy improves memory & pathology in an Alzheimer’s mouse model (aged 5×FAD/PS19 mice). They built a single-nucleus RNA sequencing dataset by combining published data from 3 independent studies, revealing both shared and cell-type-specific gene expression signatures in human Alzheimer’s samples; Using the cell-type-specific Alzheimer’s profiles from their integrated analysis, they screened for network-correcting drug candidates using a drug expression database generated with human cancer cell lines – the Connectivity Map (CMap) – 25 repurposable drugs were identified. Letrozole & irinotecan were prioritised & the combination of these rescued Alzheimer’s-like memory impairments in aged 5×FAD/PS19 mice with both Aβ & tau pathologies (Click here to read more about this and click here to read press summary about this research).
1. Could an answer come from exploding stars?:
Researchers found that lithium levels are significantly reduced in the prefrontal cortex of individuals with both mild cognitive impairment & Alzheimer’s across two independent cohorts. Curiously, they found that highly significant concentrations of lithium were detected inside β-amyloid plaques (a pathological hallmark of Alzheimer’s). In preclinical models, lithium deficiency accelerated β-amyloid deposition & promoted Alzheimer’s features in mouse models. Lithium supplementation with lithium orotate (rather than lithium carbonate) showed reduced amyloid sequestration & more effectively elevates non-plaque lithium in the brains of mice. The investigators report that the transcriptome of lithium deficiency broadly overlaps with the transcriptome of Alzheimer’s pathology in humans (Click here to read more about this and click here to read a press summary on this research).
2. The devil is in the detail:
New data on two mitophagy activators (novel Parkin activator (FB231) & PINK1 activator (MTK458)) finds that both act independently of PINK1/Parkin to increase mitophagy by acting as weak mitochondrial toxins, which actually reduces cell viability in the presence of mitochondrial stress. “Need for caution in screening for mitophagy activators in the presence of mitochondrial stressors because seemingly silent mitochondrial toxins may appear as promising pharmacological mitophagy potentiators”, even if initial hits were obtained using purified enzyme targets ‘sans cells’ (Click here to read more about this).
3. Exercise matters!
Using data from the Michael J Fox Foundation’s PPMI dataset (N=120 patients with early PD), researchers reported that regular physical activity is associated with a slower rate of neurodegeneration in the temporoparietal cortex & limbic areas in Parkinson’s (Click here to read more about this and click here to read a press summary on this research).
Using longitudinal analysis of PPMI blood samples, researchers investigated DNA damage in Parkinson’s and found disrupted DNA repair pathways. They observed a biased suppression of longer transcripts, indicating age-related, transcription-stalling DNA damage. At baseline, DNA damage was only detected in patients with more severe progression of motor symptoms over 3 years (potential predictor of disease severity biomarker?). These results were validated in independent Parkinson’s cohorts. Increased DNA damage was also observed in postmortem nigral dopamine neurons in PD cases (Click here to read more about this).
2. First BlueRocker in for Phase 3:
Bluerock Therapeutics announced that the first participants has been treated in their randomized, sham surgery-controlled, double-blind Phase 3 “exPDite-2” trial of the investigational cell therapy bemdaneprocel for Parkinson’s (Click here to read more about this).
3. Phase 2 results for AlzProtect:
Alzprotect & collaborators presented the results of their Phase 2a randomized trial (12 weeks) & open-label extension (6 months) exploring safety, biomarkers, & disease progression of AZP2006 (a progranulin–prosaposin stabilizer) in Progressive Supranuclear Palsy. Small study, but the trends in efficacy favour the AZP2006 treated group (Click here to read more about this).
1. Exercise people! Just do it:
Data reported at the Movement Disorder Society (MDS) 2025 meeting from the randomized controlled CYCLE-II trial (NCT04000360): researchers reported that 12-months of home-based high-intensity exercise intervention lowered the rate of motor symptom progression (according to UPDRS-3 scores) in 256 people with mild-to-mod Parkinson’s. “Cycling regimen demonstrated a significantly lower rate of motor symptom progression at 12 months than those receiving usual care, as rated by the MDS-UPDRS Part III scale (+3.7 points; P<0.0001)”; 93% adherence, with study participants completing 92.4 min exercise per week (Click here to read more about this).
2. Topline results from a new Phase 1b trial:
Herantis Pharma presented positive topline data from their Phase 1b trial of small peptide molecule HER-096 (designed to mimic the activity of cerebral dopamine neurotrophic factor; CDNF) in people with Parkinson’s at MDS2025 (Click here to read more about this).
3. CRISPR starts to stretch its legs and get results:
Using CRISPR/Cas9 (a DNA editing technique), researchers can conduct large-scale random mutation screening studies to identify to identify new genes involves in biological processes. Employing this unbiased approach, researchers identify key genes & pathways specifically implicated in the intracellular accumulation of Parkinson’s-associated alpha synuclein, including heparan sulfate proteoglycans biosynthesis & Golgi trafficking (Click here to read more about this).
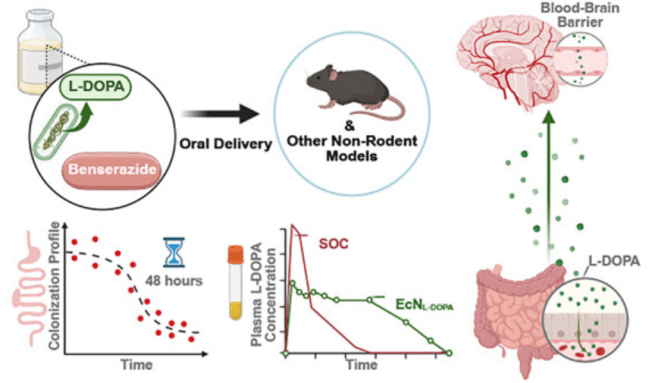
1. Continuous delivery of levodopa:
Researchers reported that oral administration (with benserazide) of gut bacteria bioengineered to produce L-DOPA could maintain therapeutic plasma L-DOPA concentrations & increased dopamine levels in the brain. The bacteria continuously produce levodopa as they transiently reside in the gut. Testing of this approach improved behavioural symptoms in a mouse model of Parkinson’s (Click here to read more about this).
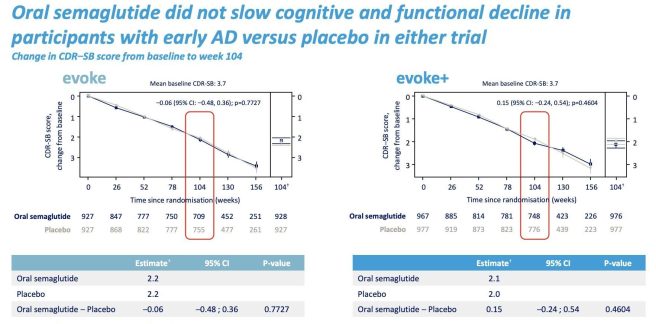
2. Semaglutide had no impact on Alzheimer’s
Novo Nordisk announced that the EVOKE and EVOKE+ Phase 3 trials of semaglutide in 3808 early-stage Alzheimer’s patient did not “translate into a delay of disease progression” even as it “resulted in improvement of AD-related biomarkers” (Click here to read more about this).
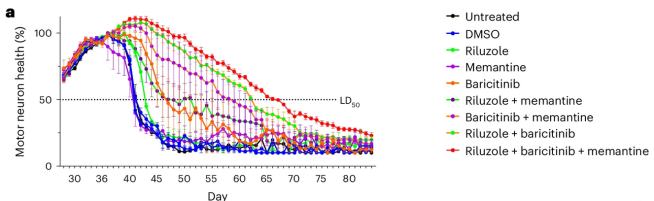
3. A combination therapy for ALS?
Combinations – all the cool kids are doing them! New research presents patient-derived iPSC models of ALS that identified baricitinib + memantine + riluzole as a promising potential therapeutic combination (Click here to read more about this).
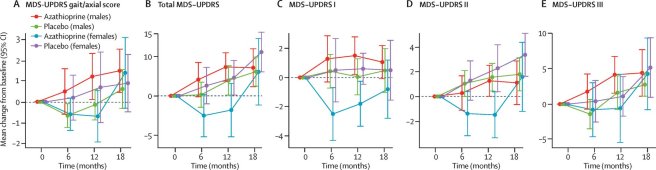
1. AZA-PD trial results:
The results of the Azathioprine for the treatment of early Parkinson’s disease (AZA-PD) study have been published; This was a randomised, double-blind, placebo-controlled, proof-of-concept, phase 2 trial. The agent was well-tolerated, but the primary outcome (UPDRS gait-axial score) was not met; Exploratory analyses suggested effects on peripheral & central immune biomarkers & on motor symptoms (interestingly more for females – this warrants further exploration (Click here to read more about this and click here to read an editorial on this research). (Cure Parkinson’s is a funder of this project)
2. A vaccine for Parkinson’s?:
AC Immune report encouraging interim data from their Phase 2 VacSYn trial of their anti-alpha-synuclein active immunotherapy (vaccine) ACI-7104.056 in early Parkinson’s; Robust antibody response, generates antibodies that access the CNS. This study (NCT06015841) involves 34 participants randomized 3:1 to receive ACI-7104.056 or placebo (20 participants have been treated for up to 18 months); They report “stabilization of disease-relevant biomarkers”, no UPDRS III OFF progression in the ACI-7104.056 group at 74 weeks (Click here to read more about this and click here to read a press summary on this research).

3. Don’t be obstructive:
An electronic health record–based cohort study finds obstructive sleep apnea appears to be an independent risk factor for the later development of Parkinson’s, but this risk could be modified by early treatment with continuous positive airway pressure (Click here to read more about this).
And that is it.
Those were some of the pieces of Parkinson’s research that grabbed our attention here at SoPD HQ in 2025. It is not an exhaustive list, and I apologise to any researchers who feel left out.
It was an extremely eventful year for Parkinson’s research with a lot of clinical trial data being released – some encouraging, others disappointing – all providing more information on experimental therapies that are being investigated for Parkinson’s.
Final thoughts:
 Source: BBC
Source: BBC
“There’s a story I’m reminded of. A forest is on fire. All the animals live terrified, helpless. But the small bird flies back and forth to the sea, back and forth carrying drops of water in this little beak. A snake laughs and asks, ‘Why bro? You will never put the fire out.’ The poor bird replies, ‘Yes, I know’. ‘Then why do you do it again and again?’, the snake asks once again. ‘I’m just doing my part’, the bird replies for the last time. The bird knows that he won’t stop the fire but it refused to do nothing. In a world that often tells us that we are too small to make a difference, that story reminds me that the power of one is not about the scale, it’s about choice. About showing up, about refusing to be silent or still when it matters most”
This quote comes from a speech that was given by Pep Guardiola at a graduation ceremony at the University of Manchester in June 2025. The Manchester City Football club coach was receiving a honorary doctorate, and as part of his lecture he gave an emotional plea regarding the humanitarian crises around the world, emphasizing the suffering of children in conflict zones like Gaza, Sudan, and Ukraine should transcend politics. Regardless of geopolitics, religion or ideology, he urged compassion.
Beyond his impassioned ask, there is a good lesson behind his words.
And as we look to what Parkinson’s research holds in 2026, the Parkinson’s community should not focus on the scale, and simply make a choice about being more engaged. In the UK, we will need the EJS ACT-PD clinical trial platform to recruit as quickly as possible to demonstrate its viability and utility. And there will be other research opportunities as well, so we need the Parkinson’s community fully involved with everything that is going on in 2026.
Given that it is such an interesting time for Parkinson’s research, it is better to be the little bird choosing to do his small part, rather than the snake doing nothing and simply waiting to see what happens.
Wishing you all a very good new year,
Simon
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE: The author of this post is an employee of Cure Parkinson’s, so he might be a little bit biased in his views on research and clinical trials supported by the trust. That said, the trust has not requested the production of this post, and the author is sharing it simply because it may be of interest to the Parkinson’s community.
The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
In addition, many of the companies mentioned in this post are publicly traded companies. That said, the material presented on this page should under no circumstances be considered financial advice. Any actions taken by the reader based on reading this material is the sole responsibility of the reader. None of the companies have requested that this material be produced, nor has the author had any contact with any of the companies or associated parties. This post has been produced for educational purposes only.
The banner for today’s post was generated by Gemini






























What was done for KJ is so beautiful. When I think about the care provided by these researchers and by his parents, expressing the importance and preciousness of a single young life, and then consider that bombs are being heedlessly dropped on children half a world away, the dissonance is almost beyond cognitive limits. In fact, it is beyond them, and you have to tune it out. But still, we know it is there.
LikeLike