|
# # # # At the end of each year, it is a useful process to take stock and review what we have learnt over the last 12 months. 2023 has been an extremely busy year for Parkinson’s research, with a lot of clinical trial results and new insights. In today’s post, we will consider three big Parkinson’s-related research takeaways of 2023 (based on our humble opinions here at the SoPD), and then we will provide an extended overview of some of the important pieces of news from the last 12 months (Be warned: this will be a rather long post). # # # # |
 Source: Reddit
Source: Reddit
2023 was a year that reminded me of Ken Burn’s quote:
“History doesn’t repeat itself, but human nature remains the same.”
Sam Clemens wrote something similar about history rhyming, but Burns is more on the mark. For the first time in three years, it felt like the heavy weight of the COVID-19 pandemic was lifted off us in 2023 and life could get back to normal.
But what is normal?
Mainstream media bombarded us with news of the ongoing war in Ukraine and then a fresh outbreak of violence in the middle east which threatens to spill over into much wider conflict. If all we did all day was keep track of these sorts of events, we would build up a pretty bleak picture of human nature.
 Ukraine or Gaza? Source: Wikipedia
Ukraine or Gaza? Source: Wikipedia
But there are other aspects to human nature that are more inspiring and can give us hope. Key among them is a desire to discover new things and perform amazing feats of scientific/engineering achievement. In 2023, these included:
- Landing a space craft on the southern pole of the moon, releasing a rover, and finding the remnants of an ocean of magma that helped form the surface of the moon (Click here to read more about this).
- Getting the first CRISPR-based treatment for sickle cell disease approved for clinical use (Click here to read more about this).
- Inventing floating ‘artificial leaves’ that can generate clean fuels from sunlight and water (Click here to read more about this).
- Growing rice in soil that had been collected on Mars and returned to Earth (Click here to read more about this).
- Launching a 400 foot (121 meter) high & 30 foot (9 meter) wide SpaceX Starship into space (Click here to read more about this).
- Developing an AI that found drugs that can combat drug-resistant infections (Click here to read more about this).
- Engineering a new non-invasive brain-reading method that is able to translate a person’s neural activity into a continuous stream of text (Click here to read more about this).
- Conducting the first evolution experiment of synthetic ‘minimal cells’ – JCVI-syn3B bacterial cells, whose genomes were trimmed to just 493 essential genes and are the smallest of any known free-living organism – and discovering that life finds a way (Click here to read more about this).
- Successfully returning samples collected by NASA’s OSIRIS-REx mission to the asteroid 101955 Bennu, back to Earth. Understand that OSIRIS-REx successfully touched down on Bennu at a distance of 200 million miles (320 million kilometers) from Earth, and Bennu was travelling at 63,000 miles per hour (101,000 kilometers per hour!!!) as it orbited the sun (Click here to read more about this).
There are many more examples of these kinds of achievements that made 2023 an amazing year, and give me hope about human nature. And here I think of another Ken Burns quote:
“I think we too often make choices based on the safety of cynicism, and what we’re lead to is a life not fully lived. Cynicism is fear, and it’s worse than fear – it’s active disengagement”
As we look to 2024, let us all be actively engaged.
Below is a list of some of the more interesting Parkinson’s research findings of the year – by month, but starting with the top three according to the team here at SoPD HQ.
|
# EDITOR’S NOTE: The author of this blog is the director of research at the medical research charity Cure Parkinson’s. For the purpose of transparency and to eliminate any sense of bias, where Cure Parkinson’s is a funder of the research it shall be noted. # |
The 3 main SOPD highlights in Parkinson’s-related research for 2023
(in no particular order – just our opinion)
1. The development of a multi-arm, multi-stage clinical trial platform for Parkinson’s:
One of the most exciting projects in the field of Parkinson’s research at the moment is the development of multi-arm multi-stage clinical trial platforms. Traditionally, when we test new therapies for a condition, we set up a trial that compares the experimental treatment to a placebo (inert/not biologically active) treatment. We conduct the study and then examine the results at the end to determine if the therapy has had any impact. There is typically a long period of time in the build up to the trial, and a long period of time following the study as the results are analysed.
An observer (I can’t recall who it was) once described the current system of clinical trials as being analogous to building a football stadium for just one game and then tearing it down again, before planning another stadium for the next game.
 Building stadiums for a single game of football. Source: WWM
Building stadiums for a single game of football. Source: WWM
Multi-Arm Multi-Stage (or MAMS) trials involve multiple agents being tested at the same time within a single trial. Each therapy is tested in one group (or arm) of participants. In this fashion, the MAMS design provides a quicker and ultimately cheaper option for testing new therapies than the conventional individual trial approach. But critically it also involves interim analyses which allow for regular checking if the drugs are working during the running of the trial.
Rather than waiting till the end of the study to find out whether a therapy actually worked, in a MAMS trial the investigators will have the data being constantly assessed by an independent monitoring group which can tell them if an agent is demonstrating any signs of efficacy.
 Source: Eupati
Source: Eupati
These regular assessments of the data as the trial is being conducted are needed so that decisions can be made about which therapies should continue to be in the trial and progress into the next phase of testing, and which should be stopped.
Below is an theoretical example of how a MAMS study could function:
 Source: iospress
Source: iospress
In the schematic provided above, 12 drugs (D1-D12) have been Phase 2 tested (or trial initiated) over the 5 year course of the study. Of these, eight have been determined to be having no effect and terminated (based on the interim analysis) while 2 (D1 and D8) have proceeded seamlessly into Phase 3 trials. Such a plan allows for 10 therapies to be Phase 2 tested in the span of 5 years.
The Edmond J Safra Accelerating Clinical Treatments for Parkinson’s Disease (EJS ACT-PD) project is one of several MAMS platforms being set up for Parkinson’s. And at Cure Parkinson’s, we’re VERY proud to be part of this effort – right from its inception (Click here to read more about this). Expect to hear a lot more about this project in 2024 and 2025 (Click here to read a previous SoPD post on this topic).
There is also a MAMS platform being developed by the Michael J. Fox Foundation for Parkinson’s Research called the Path to Prevention (P2P) platform trial, which will focus on testing drugs in people at risk of developing Parkinson’s. Using the infrastructure put in place by their Parkinson’s Progression Markers Initiative, P2p is being designed to catalyze clinical development for prodromal (pre-diagnosis) Parkinson’s.
These (and other) efforts will hopefully help to speed up the development of new therapies to slow or stop the progression of Parkinson’s.
2. New biomarkers for Parkinson’s?:
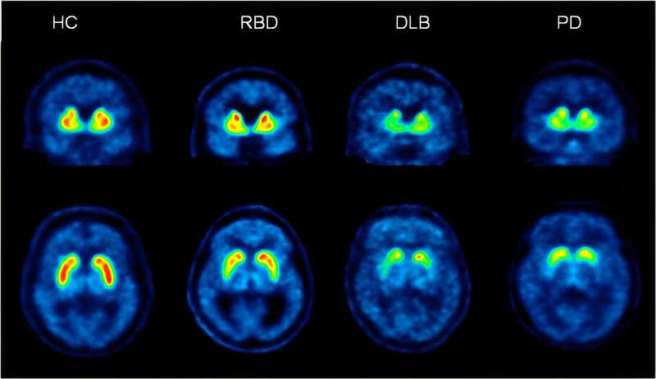
2023 should be remembered as the year that biomarker research for Parkinson’s really started to build up steam. This year we have seen three different pieces of biomarker research come to the forefront. First, researchers with funding from the Michael J. Fox Foundation for Parkinson’s Research, published results demonstrating an alpha-synuclein seed amplification assay shows promise for the identification of and early detection of Parkinson’s (Click here to read more about this, click here and here to read the summary of the research, and click here to read a SoPD post of this research).
 In 2023, researchers demonstrated that levels of a particular enzyme were extremely elevated in the cerebrospinal fluid of people with Parkinson’s, and – like the alpha-synuclein seed amplification assay mentioned above – could potentially be used as a biomarker for Parkinson’s. The enzyme is called DOPA decarboxylase, and it is used in the production of the chemical dopamine. Specifically, DOPA decarboxylase converts the chemical levodopa into dopamine (Click here to read the report and click here to read a SoPD post on this topic).
In 2023, researchers demonstrated that levels of a particular enzyme were extremely elevated in the cerebrospinal fluid of people with Parkinson’s, and – like the alpha-synuclein seed amplification assay mentioned above – could potentially be used as a biomarker for Parkinson’s. The enzyme is called DOPA decarboxylase, and it is used in the production of the chemical dopamine. Specifically, DOPA decarboxylase converts the chemical levodopa into dopamine (Click here to read the report and click here to read a SoPD post on this topic).

The production of dopamine. Source: Slideplayer
And then a third group of researchers have demonstrated that the assessment of damage to mitochondrial DNA could also be used as a potential biomarker for Parkinson’s. Mitochondria serve as the power stations for cells, providing them with energy to do their tasks. They contain their own DNA, and the researchers have found that in Parkinson’s that mitochondrial DNA displays higher levels of damage (compared to people without Parkinson’s – Click here to read the report and click here to read a SoPD post on this topic).
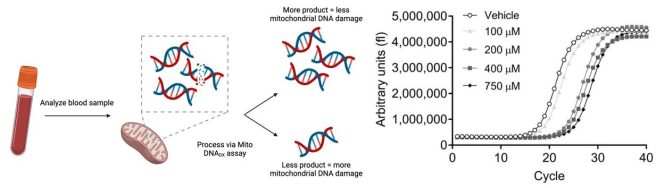 Source: PMC
Source: PMC
These three streams of biomarker research require additional work in order to better refine their potential utility, but they point towards a future where Parkinson’s will no longer be a clinically diagnosed disease, but rather a biochemically defined condition.
3. Not just a lysosomal enzyme:
Glucocerebrosidase (GCase) is an enzyme that is involved in the waste disposal systems of cells. It helps to break down the waste inside tiny cellular rubbish bags called lysosomes. People with Parkinson’s have been reported to have lower levels of GCase enzyme activity, and tiny errors in the section of DNA that provides the instructions for making GCase (a region referred to as the GBA1 gene) have been found to be the #1 genetic risk factor for developing Parkinson’s (occurring in 10-15% of people with PD).
GCase had always been associated with lysosomal function, and as such much of the research in this area had focused its attention on the lysosomes. But in 2023, a group of researchers conducted an elegant study where they looked at which proteins GCase interacts with inside of cells, and they found that 20% of the top 100 interactor proteins were specific to mitochondria function (rather lysosomes). Further analysis found that GCase is also imported into mitochondria & interacts with various mitochondrial proteins, influencing energy metabolism in cells (Click here to read more about this and click here to read an SoPD post on this research).
This research was fascinating as it showed that many of our assumptions about Parkinson’s need to be stress tested and there may be important details that we are missing in our understanding about the biology underlying the condition. I look forward to following this research further, particularly if some of the GCase activator agents (like ambroxol) being developed for Parkinson’s are found to increase transportation of GCase to the mitochondria and improve their function.
# # # # # # #
The three pieces of research news above were what grabbed my attention the most in 2023, but it was a very full year of new data and findings. Below, we’ll go month-by-month and discuss some of the other highlights.
Let’s begin with:
1. Inhibikase publishes preclinical data:
The biotech company Inhibikase Therapeutics (with Johns Hopkins collaborators) presented data in preclinical models of Parkinson’s of their brain-penetrant, oral c-Abl inhibitor IkT-148009 which is currently in clinical trial (NCT05424276). The data indicates that IkT-148009 is neuroprotective even when treatment began 4 weeks after initiation of the neurodegeneration (Click here to read more about this).
2. The inhibition of CPT1:
Researchers pointed towards inhibition of carnitine palmitoyl-transferase 1 (CPT1 – a regulator of key step in the metabolism of long-chain fatty acids) as a potential target in Parkin (PARK2)-associated Parkinson’s. They reported that inhibition of CPT1 alleviates motor & non-motor issues in PARK2 mutant mice (Click here to read more about this).
 3. Viral associations go viral:
3. Viral associations go viral:
Researchers identified 45 viral exposures significantly associated with increased risk of neurodegenerative disease in a large medical dataset, and then replicated 22 of these associations in a second large dataset. They replicated the previously reported association between Epstein-Barr & multiple sclerosis, AND they reported influenza and pneumonia were significantly associated with Alzheimer’s and Parkinson’s (Click here to read more about this).
1. Remote recording and stimulation:
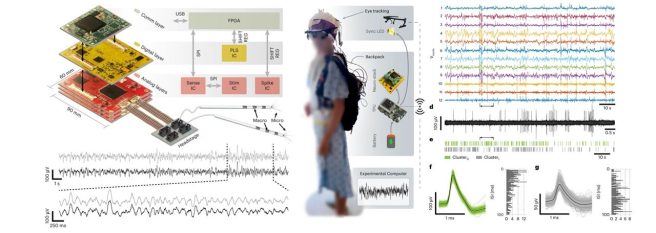
Researchers presented a wearable platform for closed-loop stimulation & recording of single-neuron & local field potential activity in freely moving humans. Current technologies that record single-neuron activity are bulky, costly and require a person to be immobile. But now researchers present “Neuro-stack” – a handheld-sized device that can record and stimulate single-neuron activity in humans. Could this have implications for Parkinson’s? (Click here to read more about this).
2. Genomics/proteomic drug screen:
Integrating genetic & proteomic data from brain & blood, researchers highlighted potential drug targets for neurodegenerative conditions. In the case of Parkinson’s, they indicated CD38, DGKQ, GPNMB, & SEC23IP as candidate targets (Click here to read more about this report and click here to read a previous SoPD post about GPNMB).
3. Hopamine (‘’hope of mine”)
A nice piece on a different type of personalized medicine for people with Parkinson’s; Offers recommendations on how medical professionals can introduce it in daily clinical practice (Click here to read more about this).
1. Another GLP-1 receptor agonist clinical trial announced results:
The biotech firm Neuraly announced topline results of their randomized, double-blind, placebo-controlled trial designed to assess safety, tolerability, & efficacy of their GLP-1 receptor agonist NLY01 in 255 individuals with early, untreated Parkinson’s. The company reported that their agent (NLY01) was safe and well tolerated, but did not meet the primary or secondary endpoints of the study. Interestingly, they did say that they saw positive indications in some of the exploratory endpoints (Click here to read more about this).
2. Longitudinal study of REM Sleep Behavior Disorder:
More research results on REM sleep behaviour disorder: The International REM Sleep Behavior Disorder Study Group presented follow-up data from 28 centers of 1160 participants followed for more than 3 years; The “findings provide optimized clinical endpoints & sample size estimates to inform future neuroprotective trials” (Click here to read more about this).
3. Inflammation. Always inflammation.
Across two independent cohorts, new data suggests a relationship between systemic inflammation & dopaminergic degeneration in Parkinson’s. The data also indicates that the relationship is mainly driven by the lymphocyte count (N=211 PD & 344 de novo PD from PPMI – click here to read more about this).
1. Preclinical research from Roche for experimental GCase therapeutic:
Researchers at Roche presented the targeting of neuronal lysosomal dysfunction caused by Parkinson’s-associated β-glucocerebrosidase deficiency with an enzyme-based brain shuttle construct. The agent rescued lysosomal function in the brain of a mouse model of Gaucher’s disease (Click here to read more about this).
2. Now you see what UCB are doing?:
One of the leading experimental small molecule inhibitors of alpha synuclein for Parkinson’s is UCB0599 (currently being clinically tested in the large Phase 2 trial). In April, UCB researchers presented high-resolution structural data on its plausible mechanism of action (Click here to read more about this).

3. Fatty acid nitroalkene in models of Parkinson’s:
Researchers reported on the in vitro & in vivo neuroprotective actions of a fatty acid nitroalkene in models of Parkinson’s. It activated Nrf2-regulated gene expression & inhibited NOX2 & LRRK2 hyperactivation, providing neuroprotection (Click here to read more about this).

1. Immune system:
A large cross-sectional gut microbiota study across early Parkinson’s, REM sleep behavior disorder, first-degree relatives, & controls found that gut compositions are significantly altered in early PD & RBD. Lots of interesting insights here: More than 50% of RBD & early PD had exposure to benzodiazepines (Click here to read more about this).
2. Something in the water:
There were two major papers on pesticides and Parkinson’s in May. Firstly, a “cohort study of 340k service members found that the risk of Parkinson’s was 70% increase in Camp Lejeune veterans compared with veterans stationed at a Marine Corps base where water was not contaminated”. Increased risk associated with exposure to trichloroethylene (Click here to read more about this).
And then a report combining quantitative epidemiologic study of pesticide exposures & Parkinson’s, with toxicity screening in dopaminergic neurons derived from PD patient (iPSCs) to identify PD-relevant pesticides (Click here to read more about this).
4. GCase activator clinical trial results:
Researchers presented the results of the Phase 1B 28 days clinical trial in GBA1-associated Parkinson’s of BIA-28-6156, an oral glucocerebrosidase activator (this is the Lysosomal Therapeutics agent LTI-291 that was acquired by Bial). The agent looks safe and well tolerated, and it raises GCase levels. Phase 2 clinical testing are now being planned (Click here to read more about this).
1. UDCA in Parkinson’s (UP) study results:
The results of the double-blind, randomized, placebo-controlled clinical trial of ursodeoxycholic acid (UDCA) in Parkinson’s were published. The study involved 30 people taking UDCA (or a placebo) for 48-weeks. The study demonstrated that the agent is was safe and tolerable, but a larger trial will be needed to further evaluate the disease-modifying potential (Full disclosure: this study was supported by Cure Parkinson’s of which the author of the blog is the director of research – click here to read more about the study results, click here to read a press summary, and click here to read a SoPD post on the topic).
2. Being dead is different to being alive:
A recent medrxiv manuscript reported on the analyses of 220 brain tissue samples (from 130 people with Parkinson’s undergoing DBS), and concluded that the “Molecular signatures identified in postmortem human brain samples are inaccurate representations of disease processes occurring in the living brain” (Click here to read more about this).
3. Positive results for the BlueRock Phase 1 study:
Pharmaceutical company Bayer & BlueRock Therapeutics announced positive top-line results in the Phase I clinical trial of their investigational cell transplantation therapy, Bemdaneprocel (BRT-DA01), in Parkinson’s. The treatment was safe & well-tolerated in all 12 participants. Detailed trial data (primary & secondary endpoints) will be presented at the 2023 Movement Disorder meeting in Copenhagen (August 27 – 31st). Motor symptoms will be assessed at two years post-transplant. Planning is now underway for a Phase II study that is expected to begin enrolling patients in the first half of 2024 (Click here to read more about this).
1. Deeper insights into what is happening in the Parkinson’s brain:
New research explored blood transcriptomic signatures & molecular changes in the brains (the caudate & putamen regions) of 35 people with Parkinson’s (compared to 40 controls). The investigators also included the clinical outcomes. Many gene expression changes were common to both caudate & putamen (increased levels of proteins regulating of miRNA activity & immune response, decreased levels of proteins involved in the postsynaptic membrane, synaptic signaling, mitochondrial dynamics, & lipid metabolism. They also identified regionally distinct changes specific to caudate & putamen that were associated with dementia & levodopa-induced dyskinesia. “Later & earlier onset Parkinson’s were also molecularly distinct, even at the end of their disease course” (Click here to read more about this).
2. The MOVE-PD study results get published:
The results of the Sanofi MOVES-PD study – a randomised, double-blinded, placebo-controlled phase 2 study assessing the safety & efficacy of the glucosylceramide synthase inhibitor venglustat in 221 individuals with GBA1-Parkinson’s – were published. They found that “venglustat had a satisfactory safety profile but showed no beneficial treatment effect compared with placebo. These findings indicate that glucosylceramide synthase inhibition with venglustat might not be a viable therapeutic approach” for GBA1-PD. Despite not achieving a positive result, this study was a remarkable achievement in many ways (including, being the first really big trial of a genetic sub-type for Parkinson’s). A lot of useful lessons can be learned from this study (Click here to read more about this).

3. Comparing the immune system across five neurodegenerative conditions:
Researchers systematically assess the role of the immune system in five neurodegenerative conditions by estimating regional genetic correlations between these diseases & immune-cell-derived single-cell expression quantitative trait loci. They reported:
– Positive genetic correlation between Parkinson’s & Lewy body dementia (LBD) (at a locus on chromosome 4 [chr4:812416-1529267] containing TMEM175)
– No significant correlation for SNCA between LBD & PD
– RAB7L1 (chromosome 1) was significantly correlated with PD in naïve CD4 +T cells
RAB7L1 is a known risk locus for Parkinson’s, and it is involved in the regulation of T cell receptor signaling. Lots of interesting insights in this data (Click here to read more about this).
1. Alpha synuclein autoimmunity
Researchers reported that α-synuclein autoimmunity induces constipation & gut pathology in mice, with loss of enteric neurons that can elicit symptoms similar to prodromal Parkinson’s. Genetically engineered mice (MHCII−/− & expressing HLA-DRB1∗15:01) immunised with a fragment of alpha synuclein protein (α-syn32-46) produced α-synuclein-targeting T cells in the peripheral lymphoid organs. These T cell populations entered the gut & converted to mucosal tissue-resident memory cells found during infection/inflammation. The activation of both innate & adaptive immune responses in these α-syn32-46-immunized HLA mice may trigger enteric neurodegeneration in the lining of the gut. NOTE: No inflammation or T cell infiltration was observed in the brains of these mice (Click here to read more about this).
2. An Africa-specific genetic risk factor for Parkinson’s
ASAP Research and The Michael J Fox Foundation funded research identified a novel genetic risk factor in GBA1 in people of African ancestry (has not been seen in European populations) and “it could be a major mechanistic basis of Parkinson’s in African populations”. Over 197,000 individuals (1,488 Parkinson’s cases & 196,000 controls) were involved in the analysis. The researchers found a novel risk factor for PD (both increased risk of developing PD and lowered age at onset) in the GBA1 gene (rs3115534-G) for individuals with African ancestry (Click here to read more about this and click here to read a press summary on this research).
3. Long-term outcomes of a Parkinson’s gene therapy clinical trial
Researchers reported on the long-term (10 years+) persistent activity (transgene expression and bioactivity) following gene delivery to the nigrostriatal system from a gene therapy clinical trial for Parkinson’s. Postmortem studies on two patients with advanced Parkinson’s that survived 10 years following AAV-neurturin gene (Cere120). Neurturin is a neurotrophic factor like GDNF and scientists used engineered viruses to deliver Neurturin to the brains of people with Parkinson’s in the early 2000s (click here to read more about this).
1. Results of the first senolytic therapy trial in Alzheimer’s
Researchers reported the results of a Phase 1 feasibility trial of senolytic therapy in mild Alzheimer’s. It was a small study of just 5 participants who have been treated with 12 weeks of a dasatinib & quercetin combination treatment. The researchers found that levels of CSF IL-6 & GFAP increased, & senescence-related cytokines & chemokines reduced (Click here to read more about this and click here to read a press summary of the research).
2. The gut again…
Researchers reported that fiber deprivation & microbiome-borne curli shift gut bacterial populations, accelerated pathology in a mouse model of Parkinson’s. “We underline the importance of a balanced diet in limiting that progression. As their ability to perform activities of daily living reduced over time, patients with Parkinson’s may require special assistance to ensure an optimal diet & oversight of antibiotics’ use” (that could favor curli-producing intestinal bacteria – click here to read more about this).
4. Cholinergic loss in GBA1-associated Parkinson’s:
New research found that individuals with recently diagnosed GBA1-associated Parkinson’s exhibit more extensive cholinergic denervation compared to non-GBA-PD (& controls). Could this help to explain the more rapid progression in this subset of PD? (Click here to read more about this).
1. Monitoring progression in Parkinson’s:
Researchers reported that over the 26-month period, brain imaging of the activity of vesicular monoamine transporter 2 (VMAT2) is able to detect the progression of nigrostriatal degeneration (assessed across three regions) in 26 participants with Parkinson’s (Click here to read more about this and click here to read the press summary).
2. The preclinical quality, safety, & efficacy data for STEM-PD:
Generating the preclinical quality, safety, & efficacy data for a human embryonic stem cell-derived product for the treatment of Parkinson’s is a major undertaking. A research report in October looked behind the curtain of the work leading up to the STEM-PD trial. The report also outlined the plan for the STEM-PD trial: An open label study involving 8 participants with moderately advanced Parkinson’s; Dyskinesias are an exclusionary due to risk of graft induced dyskinesia; Immunosuppression for 12 months post-grafting (Click here to read more about this).
3. Renin-angiotensin medication, corticosteroids, & vaccines:
A registry-based cohort study of the entire Norwegian pop. between 2004–2019 (600M prescriptions) found renin-angiotensin medication, corticosteroids, & vaccines associated with a decreased risk of Parkinson’s. 31 drug classes associated with risk change. “Drug classes used to treat symptoms related to prodromal signs of Parkinson’s such as constipation, urological issues, & depression were associated with an increased risk of subsequent diagnosis of PD” – could monitoring of prescription history help identify folks at risk? (Click here to read more about this).
1. A novel target for neuroprotection in Parkinson’s:
Researchers Mission Therapeutics and Harvard University published the results of a study investigating ubiquitin-specific protease 30 (USP30). They found that mice with no USP30 appeared to be normal and that inhibition of USP30 in an alpha-synuclein mouse model of Parkinson’s protected the mice from neurodegeneration (Click here to read more about this and click here to read an SoPD post on this research).
2. Alpha synuclein is sooo 20th century:
A report published in November suggested “that the initiation of nigrostriatal dopaminergic neurodegeneration occurs independently of alpha-synuclein aggregation & can be tau mediated”. The researchers conducted postmortem analyses of the brains of people who died with and without Parkinson’s as well as some individuals who displayed early clinical sign of motor impairments. They found that tau rather than alpha synuclein was a good determinant of Parkinson’s (Click here to read more about this).
3. Where neuroprosthestics and Parkinson’s currently meets:
Researchers reported preclinical work & implantation of a neuroprosthesis in a 62-year-old male with a 30-year history of Parkinson’s. Epidural electrical stimulation of the spinal cord alleviated freezing-of-gait & other postural issues (Click here to read more about this, click here, here and here to read press summaries about this research, and click here to read an SoPD post on this research).
1. More interesting GLP-1 receptor data:
GLP-1 receptor agonists have long been associated with having anti-inflammatory effects. Research published in December reported that GLP-1 receptor activation reduces the induction of inflammation in the blood. Curiously, these actions are not mediated by hematopoietic or endothelial GLP-1 receptors, but rather require the presence of central neuronal GLP-1 receptors. Further evidence of the close interaction between the gut and the brain, and highlighting the need for GLP-1 receptor agonists to be able to access the brain in order to have beneficial effects in dampening down inflammation (Click here to read more about this).
2. The impact of PARIS on NRF2:
The NRF family are proteins that play a key role in managing the anti-oxidant abilities of a cell. Researchers have been exploring which proteins the NRF family members interact with and in December they reported an interesting discovery: The Parkinson’s-associated protein Parkin-interacting substrate (PARIS) is a repressor of NRF2. The scientists reported that PARIS physically associates with NRF2, suppressing of NRF2-driven transcription, and increasing oxidative stress & apoptosis within cells. This could have important implications for Parkinson’s and further justifies the identification of PARIS inhibitors (Click here to read more about this).
3. Interesting data for istradefylline:
Using Japanese electronic health record data (n=4026 Parkinson’s patients), researchers presented results suggesting the adenosine A2A receptor antagonist, istradefylline, may slow progressive levodopa daily dose increases (especially for ≥600 mg/day Ldopa). “The results of this real-world analysis suggest that treatment with istradefylline may reduce the need for increases in levodopa dosage in Parkinson’s patients over a period of several years. Further investigations concerning the mechanism underlying any potential effect of istradefylline on the rate of increase in levodopa daily dose as well as the clinical consequences of long-term adjunctive therapy with levodopa & istradefylline, especially in terms of motor complications, are needed” (Click here to read more about this).
And that is it. Those were some of the pieces of Parkinson’s research that grabbed our attention here at SoPD HQ in 2023.
So what does it all mean?
I don’t know about you, but I am glad to see the back of 2023.
And I look forward to a happier 2024.
The next SoPD post is usually the annual “Road Ahead” post, but unfortunately I am going to have to skip it this year. It is an extremely time consuming project to put together, and due to circumstances out of my control I simply do not have the capacity this year to provide it.
I am sorry for this, but I will hopefully be able to make up for it next year. Fingers crossed.
Happy new year to all!
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from Guideline