|
# # # # Analysing medical data at the national level can provide a means of screening for drugs that can have an impact on the progression of chronic diseases. The large datasets of millions of people offer the opportunity to see trends for particular drugs that could potentially point towards new avenues of research. Recently researchers in Norway have conducted such a study, looking at drugs that are associated with reduced mortality in Parkinson’s. Their results identify some agents that have already been identified by previous research. But one of the drugs they found is is getting folks excited… literally. In today’s post, we will review the research and consider how strong some of these associations are. # # # # |
 Source: Unsplash
Source: Unsplash
“It was a daisy in a glass of water”
This is how red-headed, fair skinned Paul Karason described the moment that his path was determined. He had been reading a new age magazine in the 1990s about the health and rejuvenating properties of colloidal silver. And “the story was that the daisy had been desiccated before it was put back in the water. And [now] it looked like a fresh-picked daisy” (Source).
Paul sent away for a sample of colloidal silver and started consuming it.
A lot of it.
At one point he was drinking at least 10 ounces (300ml) a day. And within a month he noticed changes. “The acid reflux problem I’d been having just went away completely,” he said. “I had arthritis in my shoulders so bad I couldn’t pull a T-shirt off. And the next thing I knew, it was just gone”
But it wasn’t till a friend visited him, that he noticed another really big change.
What was the change?
Before we go any further, it is important to know that there is no evidence that colloidal silver treats or prevents any medical condition (Source).
But was it does do it cause Argyria.
What is Argyria?
Argyria is a condition that causes a blue-gray discoloration of the skin, eyes, and mucous membranes due to the accumulation of silver in the body from prolonged exposure. While argyria is generally considered harmless, the discoloration is permanent.
Here is a photo of Paul Karason before (left) and years after (right) he started taking colloidal silver (no filters were involved in these photos, I kid you not):
 Both images are Paul Karason. Source: Cerebrodigital
Both images are Paul Karason. Source: Cerebrodigital
Whoa!
Whoa indeed.
Paul dies in late 2013 at the age of 62, after a heart attack led to pneumonia and a severe stroke. But he had continued using colloidal silver until his death (Source).
What a curious story. Why share it here?
Well, because quite often on the SoPD we share interesting bits of research that some readers may consider actionable (which it ISN’T!!!).
And they might do something silly without considering the potential consequences (or talking with their doctors).
The rationale for Karason’s decision to start self administering colloidal silver was not strong (based on something he read in a magazine). And it is unclear if he took any medical advice before starting his crazy regime. And while the consequences of his particular case were largely harmless (other than being referred to as “Papa Smurf” online), there are countless other stories of self-experimentation that did not end well.
 I mean, Papa Smurf kinda fits. Source: ABC
I mean, Papa Smurf kinda fits. Source: ABC
So before readers ever consider acting on anything they read on the SoPD site, please speak with your GP or a clinician who is familiar with your medical history.
Got it. Why the warning?
It felt necessary before discussing some interesting research that was published last year by researchers in Norway.
What were they researching?
Parkinson’s.
And?
They conducted a nationwide observational study using data from Norwegian health registries that was collected between 2004 and 2020. Screening through all of the data, the scientists were interested in identifying drugs that were associated with reduced mortality risk at 8 years post diagnosis.
This is the report from their study:
 Title: Association of Medication Use and 8-Year Mortality Risk in Patients With Parkinson Disease: Drug-Wide Trial Emulation.
Title: Association of Medication Use and 8-Year Mortality Risk in Patients With Parkinson Disease: Drug-Wide Trial Emulation.
Authors: Tuominen JA, Riise T, Romanowska J, Flores-Torres MH, Cortese M, Scherzer CR, Bjornevik K, Igland J.
Journal: Neurology. 2025 Aug 12;105(3):e213783.
PMID: 40644656 (This report is OPEN ACCESS if you would like to read it)
In their analysis, the researchers identified 14,289 individuals who had been diagnosed with Parkinson’s (the average age at diagnosis was 72 years, and 59% of the cases were male). When the investigators looked at the medical records, they identified 23 drugs associated with reduced mortality risk at 8 years.
What does it mean: “reduced mortality at 8 years”?
Because disease progression is extremely difficult to measure, the researchers used a metric that is absolute.
Death?
Exactly. So the researchers were looking for drugs that were prescribed and subsequently associated with fewer deaths in a given period of time (8 years in this case) compared to deaths in people not taking the drugs.
In order to see strong, long-term trends, the investigators only looked at drugs that were prescribed to a minimum of 100 individuals (which gave them a total of 219 drugs) and importantly, the prescription for the target drug could not have been made in the past 2 years (2 years was considered too shorter time frame to see any trend).
As mentioned above, in their dataset, 14,289 individuals were diagnosed with Parkinson’s. After diagnosis, 14,059 of these cases initiated at least one non-antiparkinsonian drug. This was the pool of drugs that the researchers focused on. There were 219 individual non-antiparkinsonian drugs identified.
The investigators looked at how many people taking each of the 219 drugs had died each year over the 8 year time frame of their study. They then compared these numbers with the numbers for deaths among 10,000 randomly selected people who had not been prescribed the drug. And using this approach, they found 23 drugs that were associated with reduced levels of death during the 8-year period.
The 23 drugs (and their normal disease indication) were:
- Ranitidine (a histamine-2 blocker – used for indigestion, heartburn and acid reflux)
- Pantoprazole and esomeprazole (proton pump inhibitors – used in the short-term treatment of reflux symptoms)
- Losartan (an angiotensin receptor blocker – used to treat high blood pressure and manage heart failure)
- Atorvastatin (a statin medication used to prevent cardiovascular disease and reduce high cholesterol)
- Tadalafil (a phosphodiesterase 5 inhibitor used to treat erectile dysfunction, benign prostatic hyperplasia, and pulmonary arterial hypertension)
- Levothyroxine sodium (thyroid hormone used to treat hypothyroidism (underactive thyroid))
- Phenoxymethylpenicillin, erythromycin, and azithromycin (antibiotics used to treat a number of bacterial infections)
- Piroxicam, meloxicam, naproxen, and glucosamine (nonsteroidal anti-inflammatory drugs – “NSAID” – used to treat pain and help relieve symptoms of arthritis)
- Combined codeine/paracetamol and tramadol (opioid analgesics used to treat moderate to severe pain)
- Zopiclone and melatonin (sleep aids used for the short-term management of insomnia)
- Mianserin (a tetracyclic antidepressant used to treat depression and anxiety)
- Mometasone (a nasal corticosteroid (specifically, glucocorticoids) used to treat certain skin conditions, hay fever, and asthma)
- Ethylmorphine with and without a combined expectorant (an opioid analgesic and antitussive used in treating coughs)
- Dexamethasone (fluorinated glucocorticoid medication used to reduce inflammation and suppress the immune system for various conditions)
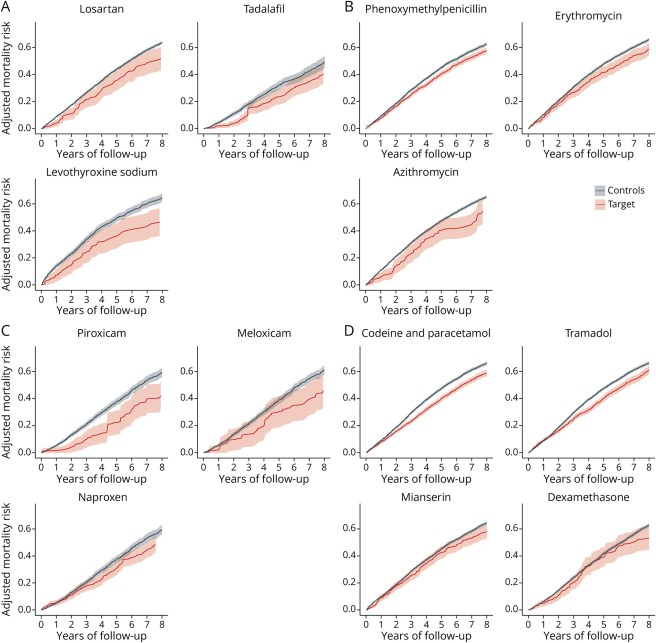
In the report, the researchers present the agents in graphs demonstrating the risk of mortality over time (the black line in the graphs are PD cases that did not use the medication (“Controls”), while the red line indicates those PD cases that were exposed to the medication post PD diagnosis (“Target”) – you will note that all of the red lines are below the black lines in the graph, suggesting a reduced risk of mortality based on exposure to the drug in question):
Source: PMC
The researchers concluded the report of their study by writing “these findings are exploratory and, therefore, insufficient to justify immediate clinical application, they warrant further investigation and potential inclusion in clinical trials”.
Interesting. Has anyone every reported similar results before?
The nonsteroidal anti-inflammatory drugs (NSAID) are not surprising, and we have previously discussed some of the research on this class of drugs here on the SoPD website (Click here to read that post). Likewise, dexamethasone has previously been proposed and we have looked at this agent as well (Click here to read a previous SoPD post on this topic). And losartan is interesting as the broader class of sartan drugs have been associated with reduced risk of Parkinson’s (Click here to read a previous SoPD post of this topic) and it’s close cousin Telmisartan will be one of the first drugs tested on the EJS ACT-PD multi-arm, multi-stage clinical trial platform.
Will any of them turn me blue?
No, but this isn’t a topic to joke about.
As I am always saying, I am not a clinician, just a research scientist, and I can not give medical advice. But there are some important warnings to flag here.
Chronic use of NSAID can cause kidney and liver damage, as well as bleeding in the stomach and bowels. There are also concerns that over use of these drugs increase risk of heart attack and stroke (Source).These drugs are designed for short term use in reducing pain and inflammation. They have not been tested in terms of long-term/chronic treatment. So while they won’t turn you blue, self experimenting with them is a REALLY bad idea.
Long-term treatment with dexamethasone is simply a none starter. Long-term treatment with glucocorticoids (like dexamethasone) are well known to associated with serious side effects.
And people with Parkinson’s need to be very careful playing with hypertension treatments like losartan. Many people with Parkinson’s have low blood pressure, and taking a drug like this will only lower that blood pressure further – which could lead to light-headedness or dizziness, increasing the risk of falls.
Ok, so why are we discussing this research?
Because one of the drugs in the list caught my attention and made me think “oh boy”.
Which drug?
Tadalafil.
Remind me, what does it do?
It is a phosphodiesterase type 5 (PDE5) inhibitor that is used to treat pulmonary arterial hypertension, benign prostatic hyperplasia, and erectile dysfunction.
You may have heard of its close cousin sildenafil.
Also known as “Viagra”.
 Tadalafil and sildenafil. Source: Medexpress
Tadalafil and sildenafil. Source: Medexpress
Oh boy indeed. Was Viagra mentioned in the study?
Not that I could find, and this could be because of the differences between the two drugs. Tadalafil has a longer half-life than sildenafil. This means that tadalafil hangs around in the body longer than sildenafil (the “effect” of sildenafil only lasts 4–6 hours, while tadalafil can last up to 36 hours).
Properties like this could have an impact.
So I should start taking Tadalafil? I’m up for that!
No.
Why not?
Because there is very little additional evidence supporting the testing of phosphodiesterase type 5 inhibitors as a treatment for Parkinson’s.
We have previously discussed phosphodiesterase inhibitors on the SoPD website (Click here to read that post), and while the type 5 inhibitors have been tested in preclinical models, they have not provided much encouraging data to date in terms of disease modification potential.
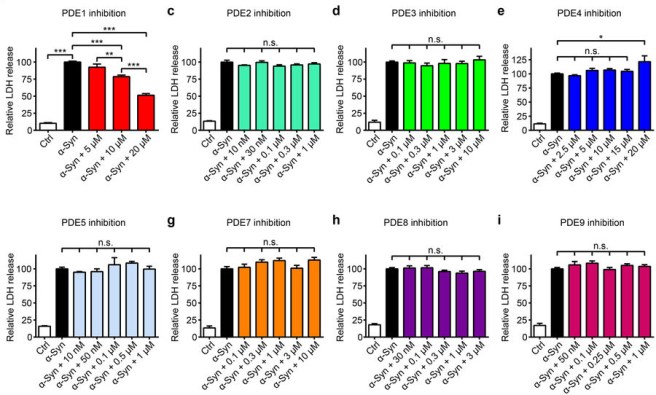
In one preclinical study, the investigators conducted a systematic analysis of other phosphodiesterase inhibitors, to try and identify a specific phosphodiesterase inhibitor that could most potently rescue cells from alpha synuclein toxicity. Alpha synuclein being a protein that aggregates in Parkinson’s, which is believed to cause stress on cells that leads to neurodegeneration.
This was the result:
Source: Nature
In that study, only phosphodiesterase type 1 inhibition (Vinpocetine) reduced alpha synuclein toxicity. Type 5 inhibition (they used sildenafil in this study) had no effect at all. And in another study, sildenafil treatment did not protect dopamine neurons in a mouse model of Parkinson’s (Click here to read more about this).
There does appear to be some kind of symptomatic effect, but the nature of this is still unclear and is dose-dependent. A preclinical study found that sildenafil treatment could potentially increase the effect of levodopa.
This is that report:
 Title: The Effect of Chronic Treatment with the Inhibitor of Phosphodiesterase 5 (PDE5), Sildenafil, in Combination with L-DOPA on Asymmetric Behavior and Monoamine Catabolism in the Striatum and Substantia Nigra of Unilaterally 6-OHDA-Lesioned Rats.
Title: The Effect of Chronic Treatment with the Inhibitor of Phosphodiesterase 5 (PDE5), Sildenafil, in Combination with L-DOPA on Asymmetric Behavior and Monoamine Catabolism in the Striatum and Substantia Nigra of Unilaterally 6-OHDA-Lesioned Rats.
Authors: Lorenc-Koci E, Kamińska K, Lenda T, Konieczny
Journal: J. Molecules. 2024 Sep 11;29(18):4318.
PMID: 39339313 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers tested the combination of levodopa and different doses of sildenafil in a neurotoxin-based rodent model of Parkinson’s. They found that a single dose (acute administration) made the motor response of levodopa more robust – not necessarily a good thing as this might exacerbate levodopa-induced dyskinesias (and there have been case study reports of this – click here to read more about it).
But chronic (regular) administration of the combination of levodopa and sildenafil had no effect, and in the case of a low dose of sildenafil, it actually reduced the effect of levodopa. So long-term use of sildenafil (which would be required for any possible disease modification in Parkinson’s) may be challenging in terms of getting the dose and dosing regime correct and managing levodopa treatment.
It all needs further research.
Has sildenafil or tadalafil ever been clinically tested in Parkinson’s?
Clinical testing of sildenafil has been conducted, primarily to determine if the agent is safe in people with Parkinson’s who are also experiencing erectile dysfunction.
An example of one of these studies:
 Title: Treatment of erectile dysfunction with sildenafil citrate (Viagra) in parkinsonism due to Parkinson’s disease or multiple system atrophy with observations on orthostatic hypotension.
Title: Treatment of erectile dysfunction with sildenafil citrate (Viagra) in parkinsonism due to Parkinson’s disease or multiple system atrophy with observations on orthostatic hypotension.
Authors: Hussain IF, Brady CM, Swinn MJ, Mathias CJ, Fowler CJ.
Journal: J Neurol Neurosurg Psychiatry. 2001 Sep;71(3):371-4.
PMID: 11511713 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers recruited 24 men with erectile dysfunction. 12 of them had been diagnosed with Parkinson’s, while the remaining 12 had been diagnosed with Multiple Systems Atrophy (MSA – click here to read a previous SoPD post on MSA).
Sidenafil was efficacious in both cohorts with a significant improvement in their ability to achieve and maintain an erection and improvements in quality of sex life were also reported. Of note/concern: the treatment did unmask or exacerbate hypotension in the MSA participants, resulting in a drop in blood pressure.
These reports have demonstrated that sildenafil can be used by men with Parkinson’s, but the investigators recommend measuring lying and standing blood pressure before prescribing sildenafil to men with parkinsonism (given that differentiating MSA from Parkinson’s is challenging in the early stages of both conditions).
There was a clinical trial evaluating whether sildenafil is effective in reducing dyskinesias in patients with Parkinson’s, but that study was terminated due to “not enough participants” (Click here to read more about this study).
But that is basically it.
As I said, there isn’t a lot of research on PDE5 inhibitors in Parkinson’s. So it is difficult to interpret the Norwegian medical records results.
In their report the Norwegian researchers actually propose an alternative reason for tadalafil popping up in their data. They suggest that “the use of tadalafil in the Parkinson’s population may reflect better overall health and survival, rather than a genuine causal effect. By contrast, in the general population, where tadalafil use was associated with higher mortality risk, use of tadalafil may reflect poorer health“.
So perhaps there is nothing to get “excited” about?
Well,…
Maybe not for Parkinson’s….
But Alzheimer’s might be a different story.
What do you mean?
In 2021, this report was published:
 Title: Endophenotype-based in silico network medicine discovery combined with insurance record data mining identifies sildenafil as a candidate drug for Alzheimer’s disease.
Title: Endophenotype-based in silico network medicine discovery combined with insurance record data mining identifies sildenafil as a candidate drug for Alzheimer’s disease.
Authors: Fang J, Zhang P, Zhou Y, Chiang CW, Tan J, Hou Y, Stauffer S, Li L, Pieper AA, Cummings J, Cheng F.
Journal: Nat Aging. 2021 Dec;1(12):1175-1188.
PMID: 35572351 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers conducted a retrospective case-control epidemiological analysis of MarketScan Medicare insurance claims data from 7.23 million individuals (2012 to 2017) and they found that sildenafil (Viagra) “usage was significantly associated with a 69% reduced risk of Alzheimer’s“.
What? Has anyone replicated this finding?
Yes. Researchers in London found a similar result when they analysed the medical records of 269,725 men, with 1,119 newly diagnosed with Alzheimer’s and they found the same result.
This is the report:
 Title: Phosphodiesterase Type 5 Inhibitors in Men With Erectile Dysfunction and the Risk of Alzheimer Disease: A Cohort Study.
Title: Phosphodiesterase Type 5 Inhibitors in Men With Erectile Dysfunction and the Risk of Alzheimer Disease: A Cohort Study.
Authors: Adesuyan M, Jani YH, Alsugeir D, Howard R, Ju C, Wei L, Brauer R.
Journal: Neurology. 2024 Feb 27;102(4):e209131.
PMID: 38324745 (This report is OPEN ACCESS if you would like to read it)
The researchers reported that use of PDE5 inhibitors was associated with a ~20% reduction in risk of Alzheimer’s, but this associated risk decreased to 44% in individuals issued more than 20 prescriptions.
Has this effect been seen in women?
Given that PDE5 inhibitors are used by women in the treatment of pulmonary arterial hypertension, researchers have looked to see if the reduced risk of Alzheimer’s also occurs in females.
But here is where the results get messy.
 Title: No association between initiation of phosphodiesterase-5 inhibitors and risk of incident Alzheimer’s disease and related dementia: results from the Drug Repurposing for Effective Alzheimer’s Medicines study.
Title: No association between initiation of phosphodiesterase-5 inhibitors and risk of incident Alzheimer’s disease and related dementia: results from the Drug Repurposing for Effective Alzheimer’s Medicines study.
Authors: Desai RJ, Mahesri M, Lee SB, Varma VR, Loeffler T, Schilcher I, Gerhard T, Segal JB, Ritchey ME, Horton DB, Kim SC, Schneeweiss S, Thambisetty M.
Journal: Brain Commun. 2022 Oct 4;4(5):fcac247.
PMID: 36330433 (This report is OPEN ACCESS if you would like to read it)
In this study – which is smaller than the previous two studies – the researchers compared incidence of Alzheimer’s and related dementia after PDE5 inhibitor initiation versus endothelin receptor antagonist initiation among patients with pulmonary hypertension. Endothelin receptor antagonists (ERA) are a standard treatment for this condition.
They identified 9968 PDE5 inhibitor initiators and 3053 ERA initiators who met our study criteria. The average age was 74 years and 69% of the cases were women. But when they looked at the incidence of Alzheimer’s over time, they observed no reduced risk.
Despite this mixed result, there are ongoing efforts to clinically test PDE5 inhibitors in Alzheimer’s. There is a biotech company called ARIBio that is developing PDE5 inhibitors for dementia.
 The company has a PDE5 inhibitor called AR1001 that has been Phase 2 tested.
The company has a PDE5 inhibitor called AR1001 that has been Phase 2 tested.
These are the results:
 Title: A phase 2 randomized, placebo-controlled study on the efficacy and safety of AR1001, a phosphodiesterase-5 inhibitor, in patients with mild-to-moderate Alzheimer’s disease.
Title: A phase 2 randomized, placebo-controlled study on the efficacy and safety of AR1001, a phosphodiesterase-5 inhibitor, in patients with mild-to-moderate Alzheimer’s disease.
Authors: Greeley D, Nash M, Herskowitz B, Kim F, Rock J, Prins N, Kim S, Xi T, Busam JA, Tete B, Choung JJ, Sha SJ.
Journal: J Prev Alzheimers Dis. 2025 Nov;12(9):100337.
PMID: 40912996 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers randomly assigned 210 individuals with mild-to-moderate dementia to once daily oral administration of placebo, 10 mg AR1001, or 30 mg AR1001 for 26 weeks. This blinded treatment period was followed by 26 weeks of optional extension treatment in which everyone was placed on the AR1001 drug.
The treatment was found to be safe and well tolerated, but the primary efficacy endpoints (improvement in ADAS-Cog 13 and ADCS-CGIC tests) were not met after 26 weeks of treatment. The length of the treatment period was rather short and it is interesting to note that the participants receiving the highest dose (30 mg) of AR1001 showed favorable trends in terms of some of the biomarkers used in the study.
And this seems to have encouraged the researchers to continue testing the drug. ARIBio currently has a Phase 3 study ongoing (the “Polaris-AD” trial) with 1535 participants involved. The 52 week study is scheduled to complete in the middle of 2026, so perhaps we will learn the results before the end of the year (Click here to read more about this).
So what does it all mean?
I have desperately tried to avoid the use of any inappropriate puns in this post. But I have to admit that my eyebrows rose when I saw the results of the Norwegian study.
And before everyone races out and starts loading up on packets of viagra, it is important to appreciate that epidemiological data involves “associations”. And association does not mean “causation”. So we must be careful in how we interpret any findings coming from these epidemiological studies.
The observation that a long-acting PDE5 inhibitor reduces the risk of mortality in people with Parkinson’s is interesting. But given the limited amount of data about this class of drugs in the context of Parkinson’s, there is obviously a lot of work to be done before we can consider clinically testing these agents.
Oh, and for the record, while I am sympathetic to the very blue Paul Karason, there is absolutely no data supporting the use of colloidal silver in Parkinson’s.
So please don’t.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR S NOTE: The author of this post is an employee of Cure Parkinson s, so he might be a little bit biased in his views on research and clinical trials supported by the trust. That said, the trust has not requested the production of this post, and the author is sharing it simply because it may be of interest to the Parkinson s community.
The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken based on what has been read on the website are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Gemini


Possibly a very simple answer as to why men using Viagra had better outcomes than women. Goes back to the health benefits of exercise. Men are using this in the hope of enjoying vigorous indoor exercise….
LikeLike