|
# # # # Drug repurposing represents a more rapid way of bringing new treatments to the public than the traditional novel drug development route. It involves clinically testing therapies in new conditions for which they are not currently approved. Researchers conduct large drug screening studies to identify agents that could be repurposed and basic science experiments can also provide data to support evaluating a particular class of drug in new medical conditions. Recently, accumulating evidence has pointed towards a class of drugs called sartans as potential candidates for repurposing for Parkinson’s. In today’s post, we will discuss what sartans are and review some of new studies suggesting the case for support. # # # # |
 The sartan family photo. Source: MDPI
The sartan family photo. Source: MDPI
The “Sartans” sound like an ancient Greek tribe or Scottish clan.
Like a formidable horde of Viking brigands bearing down on some hapless village that is unaware it is about to be pillaged. One can imagine someone in said village suddenly seeing something out of the ordinary, realizes the impending disaster, and screaming “the sartans are coming! The sartans are coming!”
 Source: Lifeinnorway
Source: Lifeinnorway
But the reality is something else entirely.
Sartans are in fact a class of drugs that are widely used for the treatment of hypertension (that is: high blood pressure). They are also administered to patients with certain heart or kidney diseases. The drugs work by blocking the function of a hormone.
That hormone is called angiotensin II.
Angiotensin II has a bad habit of causing your blood vessels (the tubes that transport blood around your body) to narrow. This action can increase your blood pressure and force your heart to work harder. Not a good situation in terms of longevity.
And before we go any further it has to be said, like any marauding tribe, the sartans need to be treated with respect – they are powerful drugs and can do worse than simply making people feel dizzy if they lower the blood pressure too much.
Good to know. But what do sartans have to do with Parkinson’s?
Well, recent research indicates that this class of drugs could be interesting in the context of Parkinson’s.
What is the new research?
Before I answer that, we need to discuss the renin-angiotensin–aldosterone system.
And what is the renin-angiotensin–aldosterone system???
It is a hormone-based pathway in the body that regulates blood pressure, fluid and electrolyte balance, and systemic vascular resistance.
 Source: Researchgate
Source: Researchgate
As the label on the can suggests, the renin-angiotensin–aldosterone system is composed of three major compounds: renin, angiotensin II, and aldosterone. And the whole system kicks into action when your our blood pressure falls. When this happens, your kidneys will release the enzyme renin into your bloodstream. Renin then grabs hold of a protein hormone that is produced in the liver called angiotensinogen.
Angiotensinogen is continuously circulating in the blood, and renin functions by cutting it into two parts. One of those parts is angiotensin I. This is a physiologically inactive protein, but it acts as a precursor for angiotensin II.
Angiotensin I is converted into angiotensin II by an enzyme called angiotensin-converting enzyme (ACE) in your lungs and kidneys. And angiotensin II is then free to go on and serve its function in the kidney, adrenal cortex, arterioles, and brain by binding to angiotensin II type I and type II receptors (receptors being like light switches that proteins like angiotensin II can bind to and activate). By binding to the angiotensin II type I and type II receptors, angiotensin II causes the blood vessels to narrow.
For those keen on more complex biology, this video will provide further information about the renin-angiotensin–aldosterone system:
In order to better manage our blood pressure, therapeutics have been developed based on various parts of this pathway. The two main types of treatments are:
- ACE inhibitors
- Angiotensin receptor blockers (angiotensin II type 1 receptor inhibitors, aka “sartans”)
Both have the same effect: they reduce the constriction of blood vessels and lower blood pressure.
|
# RECAP #1: The renin-angiotensin–aldosterone system is a hormone-based pathway in the body that regulates blood pressure. Different drugs have been developed that target this pathway (such as Sartans), and are used to help reduce blood pressure. # |
Interesting. But what do ACE inhibitors or sartans have to do with Parkinson’s?
Back in 2005, a couple of reports were published that presented preclinical data suggesting a neuroprotective role for ACE inhibitors.
This was one of those reports:
 Title: Angiotensin-converting enzyme inhibition reduces oxidative stress and protects dopaminergic neurons in a 6-hydroxydopamine rat model of Parkinsonism.
Title: Angiotensin-converting enzyme inhibition reduces oxidative stress and protects dopaminergic neurons in a 6-hydroxydopamine rat model of Parkinsonism.
Authors: Lopez-Real A, Rey P, Soto-Otero R, Mendez-Alvarez E, Labandeira-Garcia JL.
Journal: J Neurosci Res. 2005 Sep 15;81(6):865-73.
PMID: 16015598
In this study, the researchers found that ACE inhibitor Captopril displayed neuroprotective properties in a neurotoxin-based model of Parkinson’s. The lesioned rodents treated with the drug showed significantly less loss in the number of dopaminergic neurons compared to placebo treated counterparts. There was also evidence of a reduction in markers of oxidative stress.
Similar results were published by other independent research groups (Click here for an example).
Soon afterwards other research groups reported that sartans were also having a neuroprotective effect. For example, this paper:
 Title: Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra.
Title: Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra.
Authors: Grammatopoulos TN, Jones SM, Ahmadi FA, Hoover BR, Snell LD, Skoch J, Jhaveri VV, Poczobutt AM, Weyhenmeyer JA, Zawada WM.
Journal: Mol Neurodegener. 2007 Jan 15;2:1.
PMID: 17224059 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers reported that the angiotensin II type 1 receptor blocker, losartan, reduced the cell death of dopamine neurons exposed to a neurotoxin by >70% in petri dishes, and significantly reduce the neuronal cell loss in a mouse model of Parkinson’s. The investigators pointed towards the angiotensin II type 1 receptor “as a potential novel target for neuroprotection“.
Interesting. But this is all mouse models and cells in petri dishes. Is there any evidence that sartans could be useful in humans?
So very recently, there have been three report published that provide some evidence in the context of humans to support the case for exploring sartans as a treatment for Parkinson’s.
The first report was a nation-wide analysis of medical data.
This is the study here:
 Title: Protective Effect of Renin-Angiotensin System Inhibitors on Parkinson’s Disease: A Nationwide Cohort Study.
Title: Protective Effect of Renin-Angiotensin System Inhibitors on Parkinson’s Disease: A Nationwide Cohort Study.
Authors: Jo Y, Kim S, Ye BS, Lee E, Yu YM.
Journal: Front Pharmacol. 2022 Mar 3;13:837890.
PMID: 35308220 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers collected healthcare claims data between 2008–2019 from the Korean Health Insurance Review and Assessment database. They wanted to investigate whether there was any increase or decrease in the risk of developing Parkinson’s in individuals taking renin-angiotensin system drugs as a result of ischemic heart disease.
Over the 10-year period of the analysis, there were 537,116 patients diagnosed with ischemic heart disease (the cohort used in the dataset was all aged ≥60 years). Within this pool of cases, the investigators used medical data from 31,114 individuals who were prescribed renin-angiotensin system inhibitors and 31,114 individuals who were not.
Of the 62,228 cases involved, 1,086 were later diagnosed with Parkinson’s.
When the investigators looked at the data, they found that renin-angiotensin system inhibitors were significantly associated with a lower risk of Parkinson’s (the adjusted hazard ratio was 0.75 and the 95% confidence interval was 0.66–0.85) compared to the non-users of renin-angiotensin system inhibitors.
What does that mean? What are hazard ratios?
A hazard ratio is a measure of how often a particular event happens in one group compared to how often it happens in another group, over time. A hazard ratio of 1 indicates a lack of association, while a hazard ratio of greater than 1 (say, 1.50) suggests an increased risk of the event, and a hazard ratio below 1 (such as 0.75) suggests a smaller risk.
A confidence interval is the range of values within which you would expect a similar set of data to fall if you redid your test. A95% confidence interval is the range within you are confident 95% of the data would fall if you were to repeat the study with a similar data set.
So people taking renin-angiotensin system inhibitors (like sartans) had a a hazard ratio below 1 , so they were less likely to develop Parkinson’s?
That is what the data indicates.
In fact, the researchers found that the reduced risk of Parkinson’s was only present in angiotensin II receptor blockers that actually accessed the brain (that is, the drugs which are known to cross the blood brain barrier – a protective membrane surrounding the brain), and this association was stronger with increased duration of use (the longer someone used the drug, the less likely they were to be diagnosed with PD).
It should be said that the investigators were very quick in their discussion to note that these findings conflicted with some previous reports that indicate no relationship between renin-angiotensin system inhibitor use and risk of Parkinson’s (Click here and here to read more about those studies). But this new study was larger and made a special effort to differentiate between renin-angiotensin system inhibitors that did and did not access the brain.
And another study published in 2021 looked at Parkinson’s progression in individuals with Parkinson’s who were taking (or not taking renin-angiotensin system inhibitors) using data from the Michael J Fox Foundation’s massive Parkinson’s Progression Markers Initiative (PPMI) database. This study found renin-angiotensin system inhibitor use was associated with delayed need to initiate levodopa treatment in individuals recently diagnosed with Parkinson’s:
 Source: PMC
Source: PMC
This Korean study above, however, was just the first of the three recent publications supporting the case for testing sartans in Parkinson’s.
The second report explores autoantibodies. Here is that study:
 Title: Angiotensin type-1 receptor and ACE2 autoantibodies in Parkinson´s disease.
Title: Angiotensin type-1 receptor and ACE2 autoantibodies in Parkinson´s disease.
Authors: Labandeira CM, Pedrosa MA, Quijano A, Valenzuela R, Garrido-Gil P, Sanchez-Andrade M, Suarez-Quintanilla JA, Rodriguez-Perez AI, Labandeira-Garcia JL.
Journal: NPJ Parkinsons Dis. 2022 Jun 14;8(1):76.
PMID: 35701430 (This report is OPEN ACCESS if you would like to read more)
In this study, the researchers were interested in whether autoantibodies to components of the renin-angiotensin system are elevated in Parkinson’s.
What are autoantibodies?
Antibodies are Y-shaped proteins that are produced by our immune system to determine what is ‘self’ and not ‘self’. They are the foundation of our defenses against the big, bad germ/bacteria world. They bind to stuff that should not be in our bodies and act as a re flag, alerting the immune system to come and remove the offending item.
 Antibodies. Source: Astrazeneca
Antibodies. Source: Astrazeneca
Autoantibodies are antibodies produced by our immune system that are directed against our own tissues. They target ‘self’.
They are naturally produced by our bodies, and intriguingly they increase with aging. But they are particularly associated with autoimmune diseases, such as Lupus.
We are not sure why we produce autoantibodies. The causes of their production vary greatly and are not well understood. In Parkinson’s, however, autoantibodies may be produced as a result of the cell death in the brain. Some of the debris resulting from the dying cells will make its way into the bloodstream, to be removed from the body. Whilst in the blood, some of that debris could trigger the immune system, thus resulting in the production of autoantibodies.
In this new study, the researchers wanted to know whether autoantibodies for angiotensin type-1 receptor and angiotensin-converting enzyme 2 may be involved in Parkinson’s. To determine this, they collected blood from 117 people with Parkinson’s as well as 106 unaffected controls. They then analysed these samples for autoantibodies. Their results indicate that both types of autoantibodies were significantly higher in the Parkinson’s group:
 Source: PMC
Source: PMC
They also found these autoantibodies were higher in the cerebrospinal fluid of people with Parkinson’s. Next, they treated dopamine neurons in cell culture with a neurotoxin (6-OHDA) and then administered angiotensin type-1 receptor autoantibodies on half of the cells. The cultures treated with both the neurotoxin and the autoantibodies displayed more cell death than those treated with just the neurotoxin, suggesting that the autoantibodies had a negative effect on the survival of the cells:
 Source: PMC
Source: PMC
The researchers repeated the cell culture study, but this time applying the angiotensin type-1 receptor inhibitor candesartan (a member of the sartan tribe) to some of the cells treated with both the neurotoxin and the autoantibodies. They found that candesartan treatment blocked the negative effect of the autoantibodies.
The investigators concluded their study by suggesting that “therapeutical strategies blocking the production, or the effects of these autoantibodies may be useful for PD treatment, and the results further support repurposing angiotensin type-1 receptor blockers (ARBs) as treatment against PD progression”
|
# RECAP #2: A nationwide study of medical records indicates that people with ischemic injury taking angiotensin II receptor blockers had a reduced risk of developing Parkinson’s. A second study found that people with Parkinson’s have elevated levels of autoantibodies for angiotensin type-1 receptor and angiotensin-converting enzyme 2, and that these autoantibodies can have a negative effect on cells in culture. # |
Ok. And what was the third recent report “supporting the case for testing sartans in Parkinson’s”?
This one:
 Title: Single-cell genomic profiling of human dopamine neurons identifies a population that selectively degenerates in Parkinson’s disease.
Title: Single-cell genomic profiling of human dopamine neurons identifies a population that selectively degenerates in Parkinson’s disease.
Authors: Kamath T, Abdulraouf A, Burris SJ, Langlieb J, Gazestani V, Nadaf NM, Balderrama K, Vanderburg C, Macosko EZ.
Journal: Nat Neurosci. 2022 May;25(5):588-595.
PMID: 35513515 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers collected RNA from 387,483 cells that were captured from the midbrain region of the brains of people who passed away with Parkinson’s (and also from unaffected control brains).
What is the midbrain?
The loss of these dopamine neurons is one of the cardinal features of the Parkinsonian brain. These dopamine neurons reside in a region of the brain called the midbrain.
 Source: Teachmeanatomy
Source: Teachmeanatomy
More specifically, the dopamine neurons populate an area of the midbrain called the substantia nigra
What is the substantia nigra?
The substantia nigra (Latin: ‘substance dark’) region of the midbrain is actually visible with the human eye on postmortem sections of brain. This is due to the production of a molecule called neuromelanin inside of the dopamine neurons. And as you can see in the image below, the Parkinsonian brain has less dark pigmented cells in the substantia nigra region of the midbrain.
 The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
Now by analysing the RNA from each individual cell (in isolation) collected from the midbrain of people with and without Parkinson’s, the investigators behind this third study were able to identify the type of cell (neurons, microglia, astrocytes, etc) each cell was. This is because certain types of cells (like neurons) produce specific types of RNA.
And based on this RNA analysis, they were also able to identify certain subtypes of cells. For example within the neuronal class of cells, they could identify the dopamine neurons (of which there were 22,048 in their sample).
It’s really clever stuff.
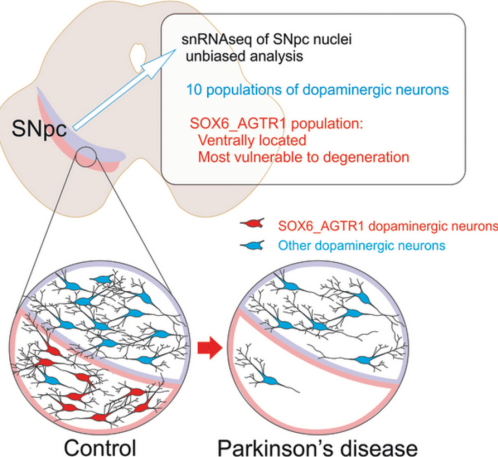
In this study, the investigators conducted a deep dive analysis into the 22,000+ dopamine neurons and identified ten different types of dopamine neurons based on the types of RNA that they produced.
But of particular interest to our discussion here is that when they looked at the Parkinson’s brains, the researchers found one of these 10 subtypes of dopamine neurons in particular was highly vulnerable to loss in the Parkinson’s brains.
That vulnerable subtype of dopamine neuron was distinguished by the production of RNA from the AGTR1 gene.
…
…
Would you like to guess what protein is produced from AGTR1 RNA?
…
Go on, take a wild stab in the dark.
…
…
The answer: AGTR1 provides the instructions for making Angiotensin II receptor Type 1 protein.
The same angiotensin…?
Yes, the same angiotensin II receptor Type 1 protein that we have discussed in the other two studies above.
So, this third report found that the dopamine neurons lost in the Parkinson’s brain produce angiotensin II receptor Type 1 protein?
Yes, that is correct.
A separate editorial written in the Movement Disorders journal provided this schematic diagram explaining the results:
Source: Movementdisorders
That editorial concludes by saying the results “further encourage the development of prodromal clinical trials for angiotensin receptor blockers (ARBS) that can cross the blood-brain barrier“.
So what does it all mean?
The papers presented in the post above provide further support to the idea that angiotensin receptor blockers (or sartans) could be an interesting candidate class of drugs for repurposing for Parkinson’s. Drug repurposing is a means of bringing new treatments to patients more quickly than the standard drug development pathway. By testing agents that have already been in clinical use, one can shave years off the amount of time required for clinical testing.
Before sartans are clinically tested in Parkinson’s, however, it would be interesting to try and identify some biomarkers that could help to zero in on the individuals who are more likely to respond to these drugs. This goal could potentially be achieved using the PPMI data in one of the studies discussed in this post. By going back and looking into the clinical data of all the individuals on angiotensin receptor blockers and then stratifying based on their rates of progression, the investigators could start looking at blood samples to see if any “responder” biomarkers exist.
As we have discussed at the top of this post, sartans are a powerful class of drugs that require respect and should not be played with. They are used in the treatment of hypertension (high blood pressure) – they are designed to lower blood pressure. People with Parkinson’s quite often have issues with low blood pressure, and taking a drug that can lower blood pressure further could be dangerous. Thus, it is not advise to take this drug outside of a well controlled clinical trial setting where blood pressure is being carefully monitored.
It would be interesting to see such a clinical trial be initiated though. There is certainly a solid body of research indicating that sartans are worth exploring.
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from singlecare.

I feel fortunate I have been taking lisinopril for 18 years. I feel like I have experienced a slower progression than I observe in others.
LikeLike