With the end of the 2016, we thought it would be useful and interesting to provide an overview of where we believe things are going with Parkinson’s disease research in the new year. This post can be a primer for anyone curious about the various research activities, and food for thought for people who may have some fresh ideas and want to get involved with the dialogue.
Never before has so much been happening, and never before has there been greater potential for real change to occur. It is a very exciting time to be involved in this field, and it really does feel like we are on the cusp of some major discoveries.
In today’s post we will outline what to expect from Parkinson’s research in 2017.
Things to look forward to. Source: Dreamstime
Before we start: something important to understand –
The goal of most of the research being conducted on Parkinson’s disease is ultimately focused on finding a cure.
But the word ‘cure’, in essence, has two meanings:
- The end of a medical condition, and
- The substance or procedure that ends the medical condition
These are two very different things.
And in a condition like Parkinson’s disease, where the affected population of people are all at different stages of the disease – spanning from those who are not yet aware of their condition (pre-diagnosis) to those at more advanced stages of the disease – any discussion of a ‘cure for Parkinson’s disease’ must be temporal in its scope.
In addition to this temporal consideration, everyone is different.
A ‘cure’ for one person may not have an impact on another person – particularly when genetics is included in the equation. Currently there is a clinical trial which is only being tested on people with Parkinson’s disease who have a particular genetic mutation (Click here to read more about this).
With all of that said, there are 4 key areas of ongoing/future research:
- Defining and understanding the biology of the condition
- Early detection
- Slowing/halting the disease
- Replacing what has been lost
EDITOR’S NOTE HERE: While we appreciate that this list does not take into account important research dealing with the improving the day-to-day living and quality of life of those affected by Parkinson’s disease (such as prevention of falling, etc), we are primarily focusing here on finding a ‘cure’.
Let’s now have a look at and discuss each of these key areas of research:
Defining and understanding the biology

Complicated stuff. Source: Youtube
The first key area of research feeds into all of the others.
It is only through a more thorough understanding of the mechanisms underlying Parkinson’s disease that we will be able to provide early detection, disease halting therapies, and cell replacement options. A better conception of the disease process would open doors in all of the other areas of research.
Given the slow pace of progress thus far, you will understand that this area of research is not easy. And it is made difficult by many issues. For example, it may be that we are blindly dealing with multiple diseases that have different causes and underlying mechanisms, but display the same kinds of symptoms (rigidity, slowness of movement and a resting tremor). Multiple diseases collectively called ‘Parkinson’s’. By not being able to differentiate between the different diseases, we have enormous confounding variables to deal with in the interpretation of any research results. And this idea is not as far fetched as it may sound. One of the most common observations within a group of people with Parkinson’s disease is the variety of disease features the group presents. Some people are more tremor dominate, while others have severe rigidity. Who is to say that these are not manifestations of different diseases that share a common title (if only for ease of management).
This complication raises the possibility that rather than being a disease, ‘Parkinson’s’ may actually be a syndrome (or a group of symptoms which consistently occur together).
Recently there have been efforts to deal with this issue within the Parkinson’s research community. We have previously written about the improved diagnostic criteria for Parkinson’s disease (click here to read that post). In addition, as we mentioned above, some new clinical trials are focusing on people with very specific types of Parkinson’s disease in which the subjects have a particular genetic mutation (Click here to read more about this). Better stratification of the disease/s will help us to better understand it. And with the signing into law of the 21 century Cures Act by President Obama, the Parkinson’s research community will have powerful new data collection tools to use for this purpose – in addition to more funding for research at the National Institute of Health (Click here to red more on this).
More knowledge of the basic biology of Parkinson’s disease is critical to the road forward. Whether the Parkinson’s disease-associated proteins, like alpha synuclein, are actually involved with the cell death associated with the condition is a question that needs to be resolved. If they are simply the bio-product of an alternative (unseen) disease process is important to know.
It is impossible to know what the new year will bring for new discoveries in the basic biology of Parkinson’s disease. Compared to 20 years ago, however, when the new discoveries were few and far between, 2017 will bring with it major new discoveries every month and we’ll do our best to report them here.
Early detection for Parkinson’s disease

Consider the impact of a pregnancy test on a person’s life. Source: Wikipedia
Ethically, the ‘early detection’ area of research can be a bit of a mine field, and for good reasons. You see, if we suddenly had a test that could accurately determine who is going to get Parkinson’s disease, we would need to very carefully consider the consequences of using it before people rush to start using it in the clinic.
Firstly there are currently no disease halting treatments, so early knowledge of future potential events may not be useful information. Second, there is the psychological aspect – such information (in light of having no treatment) may have a dramatic impact on a person’s mental wellbeing. And thirdly, such information would have huge implications for one’s general life (for example, individuals are legally bound to tell their banks and insurance companies about such information). So you see, it is a very tricky field to tackle.
Having said all of that, there are some very positive aspects to early detection of Parkinson’s disease. Early indicators (or biomarkers) may tell us something new about the disease, opening novel avenues for research and therapeutic treatments. In addition, early detection would allow for better tracking of the disease course, which would enhance our ideas about how the condition starts and changes over time.
There are numerous tests being developed – from blood tests (click here and here for posts we wrote about this topic) to saliva tests (again, click here for our post on this topic). There are even a simple urine test (click here for our post on this) and breath analysis test (click here for more on this) being developed. And there are ever increasing brain imaging procedures which may result in early detection methods (Click here for more on this).
How does the Parkinson’s research community study early detection of Parkinson’s disease though? Well, we already know that people with rapid eye movement (REM) sleep disorder problems are more likely to develop Parkinson’s disease. Up to 45 percent of people suffering REM sleep behaviour disorders will go on to develop Parkinson’s disease. So an easy starting point for early detection research is to follow these people over time. In addition, there are genetic mutations which can pre-dispose individuals to early onset Parkinson’s disease, and again these individuals can be followed to determine common ‘biomarkers’ (aspects of life that are shared between affected individuals). Epidemiological studies (like the Honolulu Heart study – click here for more on this) have opened our eyes to keep features and aspects of Parkinson’s disease that could help with early detection as well.
Slowing/halting Parkinson’s disease

Source: MyThaiLanguage
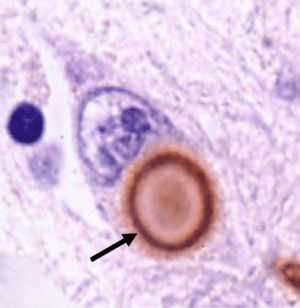
One of the most significant findings in Parkinson’s disease research over the last few years has been the discovery that transplanted dopamine cells can develop Lewy bodies over time. It is very important for everyone to understand this concept: healthy embryonic cells were placed into the Parkinsonian brain and over the space of one or two decades some of those cells began to display the key pathological feature of Parkinson’s disease: dense, circular clusters of protein called Lewy bodies.
The implications of this finding are profound: Healthy cells (from another organism) developed the features of Parkinson’s disease. And this is (presumably) regardless of the genetic mutations of the host. It suggests that the disease spreads by being passed from cell to cell. There is a very good open-access article about this in the journal Nature (click here to read that article).
Slowing down the progression of Parkinson’s disease is where most of the new clinical trials are focused. There are numerous trials are focused on removing free-floating alpha synuclein (the main protein associated with Parkinson’s disease). This is being done with both vaccines and small molecules (such as antibodies). Beyond possibly slowing the disease, whether these clinical trials are successful or not, they will most definitely provide an important piece of the puzzle that is missing: is alpha synuclein involved with the spread of the disease? If the trials are successful, this would indicate ‘Yes’ and by blocking alpha synuclein we can slow/halt the spread of the disease. If the trials fail, this would suggest that alpha synuclein is not responsible, and indicate that we need to focus our research attention elsewhere.
2017 will be very big year for Parkinson’s disease as some of these clinical trials will be providing our first glimpse at resolving this major question.
Replacing what is lost

Cell transplantation for Parkinson’s disease. Source: AtlasofScience
So if we discover a means of stopping the disease with a vaccine or a drug, this will be fantastic for people who would be destined to develop the condition… but what about those still living with the disease. Halting the condition will simply leave them where ever they are on the course of the disease – a rather unappealing situation if one is in the latter stages of the condition.
Cell transplantation is one means of replacing some of the cells that have been lost in this disease. Most of the research is focused on the dopamine neurons whose loss is associated with the appearance of the movement features of Parkinson’s disease.
Unfortunately, this area of research is more ‘blue sky’ in terms of its clinical application. It will be some time before cell transplantation has a major impact on Parkinson’s disease. And while many research groups have plans to take this approach to the clinic, there are currently just two ongoing clinical trials for cell transplantation in Parkinson’s disease:
- the Transeuro trial
- the International Stem Cell Corporation trial
The former is behind schedule due to the technical matters (primarily the source of the tissue being transplanted) and the latter is controversial to say the least (click here and here to read more). In the new year we will be watching to see what happens with a major research consortium called G-Force (strange name we agree). They are planning to take dopamine neurons derived from embryonic stem cells to clinical trials in 2018. Embryonic stem cells represents a major source of cells for transplantation as they can be expanded in a petri dish (millions of cells from just one cell). If they can be pushed in the right direction and they develop into dopamine neurons, they would allow people to start having some of the cells that they have lost to Parkinson’s disease to be replaced.
Above we have discussed the key areas of Parkinson’s disease (dealing with ‘finding a cure’) for 2017. We would love to hear your thoughts on them. If not, here on the SoPD, then somewhere else. Please get involved with the discussion in which ever forum you choose. Speak up and add your personal account of things to the discussion.
It is only through the sharing of ideas, information, and experiences that we are going to figure out this debilitating condition.
And now we are going to change focus and discuss what we are expecting/hoping for in the new year (particularly from the clinical side of things):
Bright future ahead

Looking ahead to better times. Source: Journey with Parkinson’s (Great blog!)
So looking ahead, what is happening:
Recently some major players have come together to focus on Parkinson’s disease:
- Bayer and healthcare investment firm Versant Ventures joined forces to invest $225 million in stem-cell therapy company BlueRock Therapeutics. This venture will be focused on induced pluripotent stem cell (iPSC)-derived therapeutics for cardiovascular disease and neurodegenerative disorders, particularly Parkinson’s disease (co-founders Lorenz Studer and Viviane Tabar are world renowned experts in the field of cell transplantation for Parkinson’s disease). Importantly, BlueRock has acquired rights to a key iPSC intellectual property from iPS Academia Japan, and with 4 years of funding they will be looking to make things happen (Click here to read more on this).
- Evotec and Celgene are also jumping into the IPS cell field, but they are collaborating to screen for novel drug targets. (Click here to read more on this).
- For a long time we have been hearing that the major tech company Apple is working on software and devices for Parkinson’s disease. They already provide ResearchKit and CareKit software/apps. Hopefully in the new year we will hear something about their current projects under development (Click here to read more on this).
- In February of 2016, seven of the world’s largest pharmaceutical companies signed up to Critical Path for Parkinson’s set up by Parkinson’s UK. It will be interesting in the new year to see what begins to develop from this initiative.
- Parkinson’s UK has also set up the Virtual Biotech, which is looking at providing faster means for new drugs to be brought to market. Hopefully this will take off in 2017.
In addition, there are many clinical trials starting and also announcing results. Here are the top 20 that we are keeping an eye on:
- Herantis Pharma, a Finnish pharmaceutical company, will begin recruiting 18 people with Parkinson’s disease for their Conserved Dopamine Neurotrophic Factor (or CDNF) clinical trial. CDNF is very similar to GDNF which we have previously discussed on this site (Click here for that post). Herantis will be collaborating with another company, Renishaw, to deliver the CDNF into the brain (Click here to read more on this trial).
- The results of the double-blind, placebo controlled clinical study of the diabetes drug Exenatide will be announced in 2017. We have previously discussed this therapy (click here and here for more on this), and we eagerly await the results of this study.
- AAV2-hAADC, which is a gene therapy treatment – a virus that works by allowing cells in the body other than neurons to process levodopa. The results of the phase one trial were successful (click here to read about those results) and the company (Voyager Therapeutics) behind the product are now preparing for phase 2 trials (Click here for more on this).
- Donepezil (Aricept®) is an Alzheimer’s therapy that is being tested on dementia and mild cognitive impairment in Parkinson’s disease (Click here for more on this trial).
- Oxford Biomedica is attempting to proceed with their product, OXB-102, which is a gene therapy treatment – a virus that modifies neurons so that they produce dopamine. Phase 1 successful, but did not show great efficacy. Phase 2 is underway but not recruiting (click here for more on this trial).
- Biotie is proceeding with their product, SYN120, which is new class of combination drug (dual antagonist of the serotonergic 5-HT6 and 5-HT2A receptors) which is being tested as a treatment of cognition and psychosis in Parkinson’s disease (Click here for more on this).
- Acorda Therapeutics is continuing to take the new inhalable form of L-dopa, called CVT-301 to the clinic. Phase 1 trials were successful (Click here and here to read more) and phase 2 trials are being planned.
- Related to caffeine, Istradefylline, is an A2A receptor antagonist, already approved in Japan, that is designed to reduce “off” time and suppress dyskinesias. Phase 1 testing was successful (Click here for more on this) and phase 2 trials are being planned.
- Another product from Biotie, Tozadenant, is an A2A receptor antagonist designed to reduce “off” time and suppress dyskinesias.
- UniQure was developing AAV2-GDNF – A gene therapy treatment – a virus designed to deliver GDNF (a naturally occurring protein that may protect dopamine neurons) in the brain (Click here for more on this trial). The company has recently announced cost cutting, however, and removed AAV2-GDNF from it’s list of products under development, so we are unsure about the status of this product.
- AstraZeneca are taking their myeloperoxidase (MPO) inhibitor, AZD3241, through phase 2 trials at the moment (Click here for more on this trial). Oxidative stress/damage and the formation of excessive levels of reactive oxygen species plays a key role in the neurodegeneration associated with Parkinson’s disease. MPO is a key enzyme involved in the production of reactive oxygen species. By blocking it, AstraZeneca hopes to slow/halt the progression of Parkinson’s disease.
- Genervon Biopharmaceuticals will be hopefully be announcing more results from their phase 2 clinical trial of GM 608 (Click here for more on the trial). GM 608 has been shown to protect neurons against inflammatory factors floating around in the brain. Initial results looked very interesting, though the study was very small (Click here for more on those results).
- Neurimmune (in partnership with Biogen) is proceeding with their product, BIIB054, which is an immunotherapy – an antibody that clears free floating alpha-synuclein in an attempt to halt the spread of the disease (Click here for more on this trial).
- Neuropore is continuing to move forward with their product, NPT200-11, which is a drug designed to stabilize alpha-synuclein, preventing it from misfolding and aggregating. Phase 1 trial was successful (Click here and here to read more on this). Phase 2 trials are being planned.
- Prothena are very pleased with their product, PRX002 (an immunotherapy – an antibody that clears free floating alpha-synuclein in an attempt to halt the spread of the disease (similar to BIIB054 described above)). Phase 1 trials were successful (Click here for more on this).
- Edison Pharma is currently conducting a phase 2 trial of Vatiquinone on Visual and Neurological Function in Patients with Parkinson’s Disease (Click here for more on this trial). Vatiquinone modulates oxidative stress by acting on the mitochondria on cells.
- Isradipine (Prescal®) – a calcium channel blocker that is approved for treatment of high blood pressure – is being tested in Parkinson’s disease by the Michael J Fox Foundation (Click here for more on this).
- Inosine – which is a nutritional supplement that converts to urate, a natural antioxidant found in the body – is going to be tested in a phase 3 clinical trial (Click here for more on that trial).
- In 2015, Vernalis has licensed its adenosine receptor antagonist programme (including lead compound V81444) to an unnamed biotech company. We are hoping to see the results of the phase 1 trial that was conducted on V81444 for Parkinson’s disease sometime in the new year (Click here to read more about that trial).
- And finally, we are hoping to see progress with Nilotinib (Tasigna®) – A cancer drug that has demonstrated great success in a small phase 1 trial of Parkinson’s disease. Unfortunately there have been delays to the phase 2 trial due to disagreements as to how it should be run (Click here to read more). We have been following this story (Click here and here and here to read more), and are very disappointed with the slow progress of what could potentially be a ‘game-changer’ for the Parkinson’s community. Hopefully the new year will bring some progress.
Please note that this is not an exhaustive list – we have missed many other compounds being tested for Parkinson’s disease. For example there are always alternative versions of products currently on the market being tested in the clinic (eg. new L-dopa products). We have simply listed some of the novel approaches here that we are particularly interested in.
See the Michael J Fox Foundation Pipeline page for more information regarding clinical trials for Parkinson’s disease.
EDITORIAL NOTE HERE: All of the team at the SoPD wants to wish everyone a very enjoyable festive season where ever you are. And all the very best for the new year!
Happy New Year everyone,
The team at SoPD
The banner for today’s post was sourced from Weknowyourdreams