
Exciting news this week from the world of neurodegenerative research. Researchers have identified two clinically available drugs that display neuroprotective properties.
The drugs – Dibenzoylmethane and Trazodone – are currently used to treat cancer and depression, respectively.
In this post, we will review the research and discuss what it could mean for folks with Parkinson’s disease.

Old drugs – new tricks? Source: Repurposingdrugs101
As you may have heard from media reports (for examples, click here, here and here), researchers have identified two clinically available drugs that may help in the fight against neurodegenerative conditions, like Parkinson’s disease.
The re-purposing of clinically available drugs is the focus of much attention within the Parkinson’s community as it represents a means of bringing treatments to the clinic faster. The traditional lengthy clinical trial process that is required in the development of new medications means getting a new drug to market for neurodegeneration can take up to 15 years, as the trials run over several years each (and there are three phases to pass through).

Shortening the wait. Source: Austinpublishing
In an age of smart phones and instant gratification, who has that kind of patience? ( #Wewontwait ).
Thus, re-purposing of available drugs represents a more rapid means of bringing new treatments/therapies to the Parkinson’s community.
So what is the new research all about?
This is Professor Giovanna Mallucci.

Prof Giovanna Mallucci. Source: MRC
She’s awesome.
She led the team from the Medical Research Council’s (MRC) Toxicology Unit (University of Leicester) that conducted the research and she is now based at the University of Cambridge.
Her area of research interest is understanding mechanisms of neurodegeneration, with a particular focus on prion and Alzheimer’s disease.
A few years ago, her group published this report:

Title: Sustained translational repression by eIF2α-P mediates prion neurodegeneration.
Authors: Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, Martin MG, Halliday M, Morgan J, Dinsdale D, Ortori CA, Barrett DA, Tsaytler P, Bertolotti A, Willis AE, Bushell M, Mallucci GR.
Journal: Nature. 2012 May 6;485(7399):507-11.
PMID: 22622579 (This article is OPEN ACCESS if you would like to read it)
In this study, Prof Mallucci’s group were interested in the biological events that were occurring in the brain following infection of mice with prion disease – another neurodegenerative condition. They found that a sudden loss of protein associated with the connections between neurons (those connections being called synapses) occurred at 9 weeks post infection. This led them to investigate the production of protein and they found that an increase in the levels of phosphorylation of a protein called eIF2alpha was associated with the reduction in protein synthesis occurring at 9 weeks post infection.
What is Phosphorylation?
Phosphorylation of a protein is basically the process of turning it on or off – making it active or inactive – for a particular function.

Phosphorylation of a kinase protein. Source: Nature
And what is eIF2alpha?
Eukaryotic Translation Initiation Factor 2 Alpha is (as the label on the can suggests) a translation initiation factor. This means that this particular protein functions in the early steps of the production of protein. That is to say, eIF2alpha has important roles in the first steps – the initiation – of making other proteins.
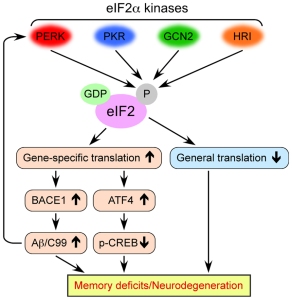
eIF2alpha’s role in neurodegeneration. Source: Frontiers
The increased phosphorylation of eIF2alpha results in the inactivation of eIF2alpha and therefore the transient shutdown of protein production.
This shutdown in protein production can serve as an important ‘checkpoint’ when a cell is stressed. By blocking general protein production, a damaged or stressed cell can have the opportunity to either recuperate or be eliminated (if the damage is beyond repair).
The shutdown can also be caused by the unfolded protein response (or UPR). The unfolded protein response is a protective mechanism triggered by rising levels of misfolded proteins.
What are misfolded proteins?
When proteins are being produced, they need to be folded into the correct shape to do their job. Things can turn ugly very quickly for a cell if protein are being misfolded or only partially folded.

Two proteins. Guess which is the misfolded protein. Source: Biogeekery
In fact, misfolded proteins are suspected of being responsible for many of the neurodegenerative conditions we know of (including Parkinson’s, Alzheimer’s, etc). Thus the unfolded protein response gives a cell time to stop protein production, degrade & dispose of any misfolded proteins, and then re-activate proteins involved with increasing the production again.
And Prof Mallucci’s group found an increase in the phosphorylation of eIF2alpha?
Yes. At 9 weeks post infection with prions, there is a decrease in the proteins required for maintaining the connections between neurons and an increase in the phosphorylation of eIF2alpha.
The interesting thing is that the researchers found that levels of phosphorylated eIF2alpha increased throughout the course of study.
So, the researchers asked themselves if promoting a recovery in protein production in the cells in neuroprotective. To test this they used a protein called GADD34, which is a specific eIF2alpha phosphatase (a phosphatase is a protein that dephosphates a protein). By introducing a lot of GADD34 in the cells, the researchers were able to re-activate eIF2alpha, rescue the connectivity between neurons and protect the cells from dying.
A cool trick, huh?
This report established the importance of eIF2alpha in the early stages of neurodegeneration, and Prof Mallucci and her group next decided to conduct a massive screening study of currently available medications to see which could be used to target eIF2alpha levels.
And that research gave rise to the report that caused so much excitement this week. This report here:

Title: Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice
Authors: Halliday M, Radford H, Zents KAM, Molloy C, Moreno JA, Verity NC, Smith E, Ortori CA, Barrett DA, Bushell M, Mallucci GR.
Journal: Brain, 2017 Epub early online publication
PMID: N/A (This article is OPEN ACCESS if you would like to read it)
The investigators began by testing 1,040 compounds (that represent many of the clinically available drugs we have) on tiny microscopic worms (called C.elegans). These worms represent a useful experimental model for screening drugs as many aspects of biology can be examined. These worms were exposed to both a chemical (called tunicamycin, which induces the unfolded protein response we were talking about above) and one of the 1040 compounds.
Of the 1040 compounds tested, the investigators selected the 20 that provided the best protection to the worms. They next analysed those top 20 compounds for their ability to reduce levels of phosphorylated eIF2alpha in cells. Cells were engineered to produce a bioluminescent signal when eIF2alpha was phosphorylated. The researchers used a potent blocker of the unfolded protein response (called GSK2606414) and a drug called ISRIB (which is an experimental drug which reverses the effects of eIF2alpha phosphorylation) as controls for the experiment.
Their results were interesting:

The results of the top 20 drugs screened. Source: Brain
As you can see from the graph above, there were five compounds (highlighted with ***) that provided a similar level of reduction as the ISRIB (control) drug:
- Azadirachtin – which is the active ingredient in many pesticides.
- Dibenzoylmethane – a cancer treatment.
- Proguanil – a medication used to treat and prevent malaria.
- Trazodone – an antidepressant used to treat depression and anxiety disorders.
- Trifluoperazine – an antipsychotic of the phenothiazine chemical class.
The investigators decided not to further investigate Azadirachtin as it is a pesticide and displays a poor ability to penetrant the blood-brain-barrier – the protective layer surrounding the brain. They also rejected Proguanil because while it is safe to use in humans, it is toxic in mice. This detail limited the amount of preclinical testing for neurodegeneration that the researchers could do. And finally Trifluoperazine was eliminated as it should not be used in the elderly populations (apparently it ‘increases the risk of death’!), which obviously limited it’s further utility given that age is a major determinant of neurodegeneration.
This selection process left the researchers with Dibenzoylmethane and Trazodone.
The researchers found that both of these drugs can cross the blood-brain-barrier and were able to prevent neurodegeneration and rescue behavioural deficits in prion-infected mice. And they observed no toxic effects of these treatments in other organs (such as the pancreas). The drugs restore correct protein production and increased the survival of the prion-infected mice.
Taking the study one step further, Prof Mallucci and her group asked if the drugs could be effective in a model of another neurodegenerative condition, such as Alzheimer’s disease. To investigated this, they treated rTg4510 mice with both of the drugs. rTg4510 mice produce a lot of a human protein (called tau) that has a particular mutation (known as P301L), which results in the onset of Alzheimer’s like pathology at an early age. The rTg4510 mice received either trazodone or Dibenzoylmethane on a daily basis from 4 months of age and were examined at 8 months of age. The researchers found significantly less cell loss and shrinkage in the brains of the mice treated with one of the two drugs when compared to rTg4510 mice that received no treatment.
The researchers concluded that “these compounds therefore represent potential new disease-modifying treatments for dementia. Trazodone in particular, a licensed drug, should now be tested in clinical trials in patients”.
As Professor Mallucci suggested to the press: “We know that trazodone is safe to use in humans, so a clinical trial is now possible to test whether the protective effects of the drug we see on brain cells in mice with neurodegeneration also applies to people in the early stages of Alzheimer’s disease and other dementias. We could know in 2-3 years whether this approach can slow down disease progression, which would be a very exciting first step in treating these disorders. Interestingly, trazodone has been used to treat the symptoms of patients in later stages of dementia, so we know it is safe for this group. We now need to find out whether giving the drug to patients at an early stage could help arrest or slow down the disease through its effects on this pathway.”
This is great for Alzheimer’s disease, but what about Parkinson’s?
Well, the researchers did not test the drugs in models of Parkinson’s disease. But we can assume that several research groups are going to be testing this drug in the near future… if they aren’t already!
But have increased levels of eIF2alpha been seen in Parkinson’s disease?
Great question. And the answer is: Yes.

Title: Activation of the unfolded protein response in Parkinson’s disease.
Authors: Hoozemans JJ, van Haastert ES, Eikelenboom P, de Vos RA, Rozemuller JM, Scheper W.
Journal: Biochem Biophys Res Commun. 2007 Mar 16;354(3):707-11.
PMID: 17254549
In this study the investigators analysed the levels of Unfolded Protein Response activation in the postmortem brains of people who passed away with or without Parkinson’s disease. Specifically, they focused their analysis on the substantia nigra (the region where the dopamine neurons reside and which is most severely affected in Parkinson’s).
The researchers found that both eIF2alpha and a protein called PERK (also known as protein kinase-like ER kinase – which phosphalates eIF2alpha) are present in the dopamine neurons in the substantia nigra of brains from people with Parkinson’s disease, but not in healthy control brains. And as the graph below shows, the investigators noted that there was a trend towards the levels of these proteins peaking within the first five years after diagnosis.

eIF2alpha & PERK levels in the brain. Source: ScienceDirect
Similar postmortem analysis studies have also highlighted the increased levels of Unfolded Protein Response activation in the Parkinsonian brain (Click here to read more on this).
The increase in Unfolded Protein Response activation could be a common feature across different neurodegenerative conditions, suggesting that trazodone and dibenzoylmethane could be used widely to slow the progress of various conditions.
Another connection to Parkinson’s disease is the finding that high levels of the Parkinson’s associated protein alpha synuclein can cause the Unfolded Protein Response:

Title: Induction of the unfolded protein response by α-synuclein in experimental models of Parkinson’s disease.
Authors: Bellucci A, Navarria L, Zaltieri M, Falarti E, Bodei S, Sigala S, Battistin L, Spillantini M, Missale C, Spano P.
Journal: J Neurochem. 2011 Feb;116(4):588-605.
PMID: 21166675 (This article is OPEN ACCESS if you would like to read it)
The researchers in this study found that introducing large amounts of alpha synuclein into cell cultures results in the initiation of the unfolded protein response. They also observed this phenomenon in genetically engineered mice that produce large amounts of alpha synuclein.
Thus, there is some evidence for eIF2alpha and unfolded protein response-related activities in Parkinson’s disease
So is there are evidence that Dibenzoylmethane might be neuroprotective for Parkinson’s disease?
Yes there is (sort of):

Title: A dibenzoylmethane derivative protects dopaminergic neurons against both oxidative stress and endoplasmic reticulum stress.
Authors: Takano K, Kitao Y, Tabata Y, Miura H, Sato K, Takuma K, Yamada K, Hibino S, Choshi T, Iinuma M, Suzuki H, Murakami R, Yamada M, Ogawa S, Hori O.
Journal: Am J Physiol Cell Physiol. 2007 Dec;293(6):C1884-94. Epub 2007 Oct 3.
PMID: 17913843 (This article is OPEN ACCESS if you would like to read it)
The investigators of this study found a derivative of dibenzoylmethane which they called 14-26 (chemical name 2,2′-dimethoxydibenzoylmethane) displayed neuroprotective functions both in cell culture and animal models of Parkinson’s disease. The researchers did not look at the unfolded protein response or eIF2alpha and PERK levels, nor did they determine if dibenzoylmethane itself exhibits neuroprotective properties.
This may now need to be re-addressed.
And is there any evidence trazodone having neuroprotective effects in other neurodegenerative conditions?
Yes.
For a review of the neuroprotective effects of trazodone (and other anti-psychotic/anti-depressant drugs) in Huntington’s Disease – Click here.
This sounds very positive for Parkinson’s disease then, no?
Weeeeeell, there is a word of caution to be thrown in here:
There have been reports in the past of trazodone causing motor-related issues in the elderly. Such as this one:

Title: Can trazodone induce parkinsonism?
Authors: Albanese A, Rossi P, Altavista MC.
Journal: Clin Neuropharmacol. 1988 Apr;11(2):180-2.
PMID: 3378227
This report was a single case study of a 74 year old lady who developed depression after losing her sister with whom she lived. She was prescribed trazodone, which was effective in improving her mood. Just several months later, however, she began presenting Parkinsonian symptoms.
Firstly the onset of a resting tremor in the left arm, then a slowing of movement and a masking of the face. The attending physician withdrew the trazodone treatment and within two months the symptoms began to disappear, with no symptoms apparent 12 months later.
And unfortunately this is not an isolated case – other periodic reports of trazodone-induced motor issues have been reported (Click here and here for examples). And this is really strange as Trazodone apparently has no dopaminergic activity that we are aware of. It is a serotonin antagonist and reuptake inhibitor (SARI); it should not affect the re-uptake of norepinephrine or dopamine within the brain.
Thus, we may need to proceed with caution with the use of Trazodone for Parkinson’s disease.
So what does it all mean?
The repurposing of old drugs to treat alternative conditions is a very good idea. It means that we can test treatments that we usually know a great deal about (with regards to human usage) on diseases that they were not initially designed for, in a rapid manner.
Recently, scientists have identified two clinically available drugs that have displayed neuroprotection in two different models of neurodegeneration. Without doubt there will now be follow up investigations, before rapid efforts are made to set up clinical trials to test the efficacy of these drugs in humans suffering from dementia.
Whether these two treatments are useful for Parkinson’s disease still needs to be determined. There is evidence supporting the idea that they may well be, but caution should always be taken in how we proceed. This does not mean that other clinically available drugs can not be tested for Parkinson’s disease, however, and there are numerous clinical trials currently underway testing several of them (Click here to read more on this).
We’ll let you know when we hear anything about these efforts.
EDITOR’S NOTE: Under absolutely no circumstances should anyone reading this material consider it medical advice. The material provided here is for educational purposes only. Before considering or attempting any change in your treatment regime, PLEASE consult with your doctor or neurologist. While some of the drugs discussed on this website are clinically available, they may have serious side effects. We urge caution and professional consultation before altering any treatment regime. SoPD can not be held responsible for any actions taken based on the information provided here.
The banner for today’s post was sourced from Linkedin
Thank you for clarifying this weeks Parkinson’s news – if only someone could do the same for politics!
LikeLike
Thanks for your message Eirwen, glad you liked the post – we’ll happily leave politics to other.
LikeLike
DBM very interesting; but not an approved drug.
Trazodone is cheap generic drug. However, approved in 1981, many PD patients have insomnia and depression and in 38 years since approved nothing to suggest stops progression of PD.
LikeLike