|
In Silicon valley (California), everyone is always looking for the “next killer app” – the piece of software (or application) that is going to change the world. The revolutionary next step that will solve all of our problems.
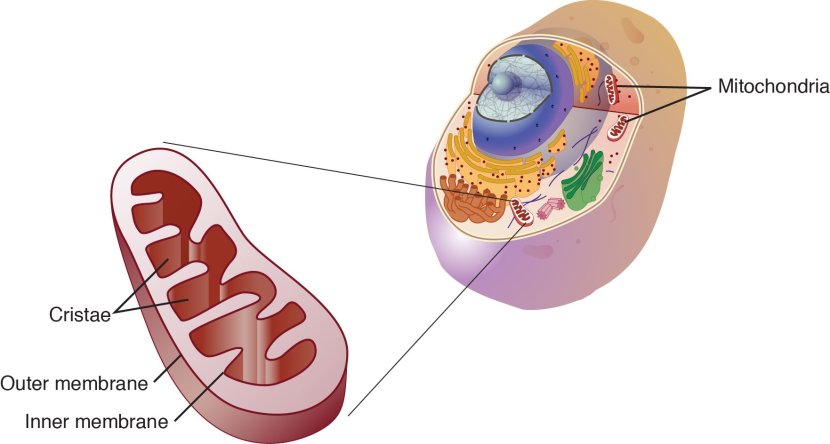
The title of today’s post is a play on the words ‘killer app’, but the ‘app’ part doesn’t refer to the word application. Rather it relates to the Alzheimer’s disease-related protein Amyloid Precursor Protein (or APP). Recently new research has been published suggesting that APP is interacting with a Parkinson’s disease-related protein called Leucine-rich repeat kinase 2 (or LRRK2).
The outcome of that interaction can have negative consequences though.
In today’s post we will discuss what is known about both proteins, what the new research suggests and what it could mean for Parkinson’s disease.
|

Seattle. Source: Thousandwonders
In the mid 1980’s James Leverenz and Mark Sumi of the University of Washington School of Medicine (Seattle) made a curious observation.
After noting the high number of people with Alzheimer’s disease that often displayed some of the clinical features of Parkinson’s disease, they decided to examined the postmortem brains of 40 people who had passed away with pathologically confirmed Alzheimer’s disease – that is, an analysis of their brains confirmed that they had Alzheimer’s.
What the two researchers found shocked them:

Title: Parkinson’s disease in patients with Alzheimer’s disease.
Authors: Leverenz J, Sumi SM.
Journal: Arch Neurol. 1986 Jul;43(7):662-4.
PMID: 3729742
Of the 40 Alzheimer’s disease brains that they looked at nearly half of them (18 cases) had either dopamine cell loss or Lewy bodies – the characteristic features of Parkinsonian brain – in a region called the substantia nigra (where the dopamine neurons are located). They next went back and reviewed the clinical records of these cases and found that rigidity, with or without tremor, had been reported in 13 of those patients. According to their analysis 11 of those patients had the pathologic changes that warranted a diagnosis of Parkinson’s disease.
And the most surprising aspect of this research report: Almost all of the follow up studies, conducted by independent investigators found exactly the same thing!
It is now generally agreed by neuropathologists (the folks who analyse sections of brain for a living) that 20% to 50% of cases of Alzheimer’s disease have the characteristic round, cellular inclusions that we call Lewy bodies which are typically associated with Parkinson disease. In fact, in one analysis of 145 Alzheimer’s brains, 88 (that is 60%!) had chemically verified Lewy bodies (Click here to read more about that study).

A lewy body (brown with a black arrow) inside a cell. Source: Cure Dementia
Oh, and if you are wondering whether this is just a one way street, the answer is “No sir, this phenomenon works both ways”: the features of the Alzheimer’s brain (such as the clustering of a protein called beta-amyloid) are also found in many cases of pathologically confirmed Parkinson’s disease (Click here and here to read more about this).
So what are you saying? Alzheimer’s and Parkinson’s disease are the same thing???
Continue reading “The next killer APP: LRRK2 inhibitors?” →