|
In Silicon valley (California), everyone is always looking for the “next killer app” – the piece of software (or application) that is going to change the world. The revolutionary next step that will solve all of our problems. The title of today’s post is a play on the words ‘killer app’, but the ‘app’ part doesn’t refer to the word application. Rather it relates to the Alzheimer’s disease-related protein Amyloid Precursor Protein (or APP). Recently new research has been published suggesting that APP is interacting with a Parkinson’s disease-related protein called Leucine-rich repeat kinase 2 (or LRRK2). The outcome of that interaction can have negative consequences though. In today’s post we will discuss what is known about both proteins, what the new research suggests and what it could mean for Parkinson’s disease. |

Seattle. Source: Thousandwonders
In the mid 1980’s James Leverenz and Mark Sumi of the University of Washington School of Medicine (Seattle) made a curious observation.
After noting the high number of people with Alzheimer’s disease that often displayed some of the clinical features of Parkinson’s disease, they decided to examined the postmortem brains of 40 people who had passed away with pathologically confirmed Alzheimer’s disease – that is, an analysis of their brains confirmed that they had Alzheimer’s.
What the two researchers found shocked them:

Title: Parkinson’s disease in patients with Alzheimer’s disease.
Authors: Leverenz J, Sumi SM.
Journal: Arch Neurol. 1986 Jul;43(7):662-4.
PMID: 3729742
Of the 40 Alzheimer’s disease brains that they looked at nearly half of them (18 cases) had either dopamine cell loss or Lewy bodies – the characteristic features of Parkinsonian brain – in a region called the substantia nigra (where the dopamine neurons are located). They next went back and reviewed the clinical records of these cases and found that rigidity, with or without tremor, had been reported in 13 of those patients. According to their analysis 11 of those patients had the pathologic changes that warranted a diagnosis of Parkinson’s disease.
And the most surprising aspect of this research report: Almost all of the follow up studies, conducted by independent investigators found exactly the same thing!
It is now generally agreed by neuropathologists (the folks who analyse sections of brain for a living) that 20% to 50% of cases of Alzheimer’s disease have the characteristic round, cellular inclusions that we call Lewy bodies which are typically associated with Parkinson disease. In fact, in one analysis of 145 Alzheimer’s brains, 88 (that is 60%!) had chemically verified Lewy bodies (Click here to read more about that study).

A lewy body (brown with a black arrow) inside a cell. Source: Cure Dementia
Oh, and if you are wondering whether this is just a one way street, the answer is “No sir, this phenomenon works both ways”: the features of the Alzheimer’s brain (such as the clustering of a protein called beta-amyloid) are also found in many cases of pathologically confirmed Parkinson’s disease (Click here and here to read more about this).
So what are you saying? Alzheimer’s and Parkinson’s disease are the same thing???
No, I’m not saying that.
They are both very different conditions, although there are some overlaps (particularly with regards to dementia – I’ll be coming back to this in an up-coming post on Lewy body dementia).
One thing for certain is that they do appear to share some common biological features. And this idea has been supported by research indicating that some Alzheimer’s disease-related proteins are directly interacting with some Parkinson’s disease-associated proteins.
Recently a research report was published that suggesting one of these interactions can have negative consequences.
This is the research report:

Title: Phosphorylation of amyloid precursor protein by mutant LRRK2 promotes AICD activity and neurotoxicity in Parkinson’s disease.
Authors: Chen ZC, Zhang W, Chua LL, Chai C, Li R, Lin L, Cao Z, Angeles DC, Stanton LW, Peng JH, Zhou ZD, Lim KL, Zeng L, Tan EK.
Journal: Sci Signal. 2017 Jul 18;10(488).
PMID: 28720718
In this study, the investigators were interested in the interactions of a Parkinson’s-associated protein called Leucine-rich repeat kinase 2 (or LRRK2).
What is LRRK2?
Also known as ‘Dardarin‘ (from the Basque word “dardara” which means “trembling”), LRRK2 is an enzyme that has many functions within a cell – from supporting efforts to move things around inside the cell to helping to keep the power on (involved with mitochondrial function).

The many jobs of LRRK2. Source: Researchgate
The gene that provides the instruction for making the LRRK2 enzyme resides on the 12th chromosome, in an area of DNA referred to as ‘PARK8’ (one of the Parkinson’s disease-associated genetic regions). It is made up of many different regions, each of which is involved with the different functions of the eventual protein.

The regions and associated functions of the LRRK2 gene. Source: Intechopen
Genetic mutations within the LRRK2 gene are recognised as being some of the most common with regards to increasing ones risk of developing Parkinson’s disease.

The structure of Lrrk2 and where various mutations lie. Source: Intech
As the image above suggests, mutations in the PARK8 gene are also associated with Crohn’s disease (Click here and here for more on this) – though that mutation is in a different location to those associated with Parkinson’s disease. And one particularly common Parkinson’s-associated LRRK2 mutation – called G2019S – is also associated with increased risk of certain types of cancer, especially for hormone-related cancer and breast cancer in women – Click here to read more about this. If you have a G2019S mutation, it is good to be aware of this association and have regular check ups.
As I mentioned above, the G2019S mutation (the name designates its location on the gene) is the most common LRRK2 mutation. In some populations of people it can be found in 40% of people with Parkinson’s disease (Click here to read more about this). But what is interesting about this mutation is that it gives rise to a LRRK2 enzyme that is hyperactive.
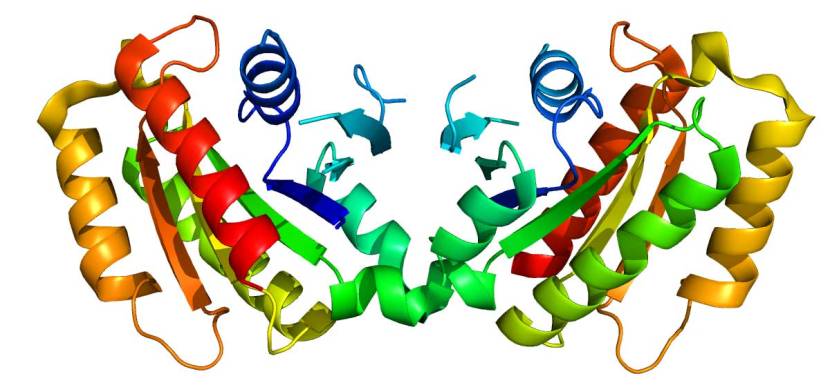
The structure of LRRK2. Source: Wikipedia
As a protein, LRRK2 interacts with many different types of other proteins, and you can imagine that in a finely balanced environment like the cells that a mutant hyperactive form of LRRK2 is going to cause problems. To get a better understanding of how these sorts of issues could relate to Parkinson’s disease, researchers have been looking at which proteins LRRK2 interacts with and bind to, and how that interaction differs in the case of a G2019S mutation.
A good example of this sort of study was published last year:

Title: Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of RabGTPases.
Authors: Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, Baptista MA, Fiske BK, Fell MJ, Morrow JA, Reith AD, Alessi DR, Mann M.
Journal: Elife. 2016 Jan 29;5. pii: e12813.
PMID: 26824392 (This article is OPEN ACCESS if you would like to read it)
In this study the researchers used a variety of screening approaches which involved analysing how proteins reacted when either normal LRRK2 or hyperactive G2019S LRRK2 were present in cells. They also used drugs called LRRK2 inhibitors (such as the pharmceutical company GlaxoSmithKline’s LRRK2 inhibitor GSK2578215A) to see which proteins are affected by reducing levels of LRRK2 activity.
This screening approach identified Rab10 as a protein affected by LRRK2 activity.
What is Rab10?
Rab10 belongs to the family of proteins called GTPases. These proteins regulate the transportation of vesicles (the small bags of necessary proteins) within a cell. The researchers went on to identify other GTPases – including Rab8a and Rab12 – as interactors with LRRK2.
The researchers found that one of LRRK2s functions is to detach these GTPases protein from associated proteins and then help them to insert into their correct position on membranes. They also found that hyperactive G2019S LRRK2 enzyme activity results in a subtle increase in this dysfunctional Rab activity which they speculated was “consistent with the long time needed for PD to manifest in humans”.
Other researchers have subsequently found addition proteins that LRRK2 interacts with.
But one of the most interesting proteins is called TAU.
What is TAU?
TAU is a microtubule-associated protein, which functions by stabilising the highways and byways inside a cell. Basically, these proteins hold together the roads which allows vesicles (the small bags I mentioned above) to be moved around the cell (by transporter proteins like kinesin).

Tau: a stabilising presence. Source: Hindawi
So why is TAU interesting?
Because it is associated with both Parkinson’s disease AND Alzheimer’s disease. In both conditions there is a build up of TAU protein which accumulation and aggregates in a similar fashion to our old friend the Parkinson’s disease-associated protein alpha synuclein (Click here for a good review article on this topic).
Now, this interaction of LRRK2 with a protein that is associated with Alzheimer’s disease got some researchers wondering:
Hmmm, I wonder if ol’ LRRK2 is interacting with some of them other Alzheimer’s-associated proteins?
And that question brings us back to the research report we are reviewing today:

Title: Phosphorylation of amyloid precursor protein by mutant LRRK2 promotes AICD activity and neurotoxicity in Parkinson’s disease.
Authors: Chen ZC, Zhang W, Chua LL, Chai C, Li R, Lin L, Cao Z, Angeles DC, Stanton LW, Peng JH, Zhou ZD, Lim KL, Zeng L, Tan EK.
Journal: Sci Signal. 2017 Jul 18;10(488).
PMID: 28720718
The investigators decided to have a look at whether there was any interaction between LRRK2 and a protein called Amyloid Precursor Protein (or APP).
What is APP?
APP is a protein that sits in the wall (or the membrane) of cells in many different types of tissue. It is particularly concentrated in neurons. Part of it is exposed to the outside world, while another part of it is inside the cell (it is a transmembrane protein). While the exact function of APP is not entirely clear, APP’s main claim to fame is that it is – as the label on the can suggests – the precursor to the perceived bad boy of Alzheimer’s disease: Beta Amyloid

APP and Beta-Amyloid. Source: Wikimedia
It has been suggested that APP plays a role memory (Click here to read more on that), and iron exporting (Click here to read more on this). But in the context of Alzheimer’s disease, the action doesn’t really start until one of three enzymes turn up.
Those enzymes are alpha, beta and gamma secretase.
Normal processing of APP by these enzymes is conducted by alpha secretase. But when Beta and gamma secretase get involved, the off-spring of that activity is the notorious Beta Amyloid, which floats off to clump together with other pieces of Beta Amyloid and these start to accumulate in the brains of people with Alzheimer’s disease.

APP processing by alpha, beta and gamma secretase. Source: CMAJ
Exactly why this occurs is unknown, though recent research suggests that Beta Amyloid may be an anti-microbial defence mechanism for the cell (Click here to read a previous post on this topic).
So what happens when LRRK2 interacts with APP?
The researchers found that LRRK2 interacted with and activates APP at very specific spot (called Thr668). This region sits on the part of the protein that lives inside the cell. It is also referred to as the intracellular domain of APP. I have highlighted it in the circled area in the image below.

Now, when LRRK2 activates APP at the Thr668 site, it results in the intracellular domain of APP detaching itself from APP and heading off to the nucleus of the cell. Unfortunately when this intracellular domain is in the nucleus, it starts to activate genes that are not exactly desirable, including some involved in apoptosis (or programmed cell death).
In a normal situation this activity is not really a problem, the cell has mechanisms that tightly regulate the apoptosis pathways. But remember that people with the G2019S mutation in the LRRK2 gene have a hyperactive version of the LRRK2 protein, which results in higher levels of the intracellular domain of APP being detached and heading off to the nucleus to cause mischief. And indeed when the researchers looked at the nucleus of cells of mice with the G2019S mutation in the LRRK2 gene, they found high levels of the intracellular domain of APP there.
An interesting result of this study is that accumulating levels of the intracellular domain of APP in the nucleus is enough to cause damage, but the hyperactive version of the LRRK2 protein enhances this effect. And the effect is bad: in aged mice that have the G2019S mutation in their LRRK2 gene, the researchers found fewer dopamine neurons than in normal mice (dopamine neurons being the cells so severely affected in Parkinson’s disease). This finding fits nicely with the slow progression of Parkinson’s disease – a gradual accumulation of the intracellular domain of APP in the nucleus eventually resulting in cell death at a late stage of life.

Lab mouse. Source: ScienceLife
The investigators next looked at what happens in humans, particularly those with a G2019S mutation in the LRRK2 gene. They did this analysis in two ways: one using induced pluripotent stem cells (or IPS cells – these are skin cells that have been turned into stem cells – click here to read more about them) and the other using postmortem brains.

Making IPS cells. Source: learn.genetics
In neurons grown from the IPS cells, the researchers found significantly increased levels of the activated APP Thr668 site in the cells from people with the G2019S mutation in the LRRK2 gene (compared to cells from people without the mutation). They also found increased levels of the activated intracellular domain of APP in the postmortem brains from people who had Parkinson’s disease with the G2019S mutation in the LRRK2 gene.
It’s a really interesting result.
Indeed. So summing up, what does it all mean?
What? No, hang on! We haven’t even got to the really good part of this study.
Que?
The most interesting part of this whole report is that the researchers completed their study by applying a LRRK2 inhibitor (LRRK2-IN-1) to the human iPS cells from people with LRRK2 G2019S mutations….and guess what happened?
They saw a reduction in the levels of the activated intracellular domain of APP!
And the investigators suggested that this led to an increase in the survival of those cells.
They also tested another LRRK2 inhibitor (a brain penetrant version called HG-10-102-01) on mice that have the G2019S mutation in their LRRK2 gene and just 24 hours later they observed a reduction in the levels of the activated intracellular domain of APP.
Wow! That does sound interesting. Have any of these LRRK2 inhibitors been tested in clinic trials?
No, not that I’m aware of (and I’m happy to be corrected on this).
‘Big pharma’ are certainly very interested in LRRK2 inhibitors though. I mentioned the GlaxoSmithKline’s LRRK2 inhibitor GSK2578215A earlier in the post, and Pfizer has also developed a highly potent, selective, brain penetrant, and in vivo active LRRK2 kinase inhibitor called 3-[4-(morpholin-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-5-yl]benzonitrile (yeah, try saying that three times really fast).

Thankfully they have conveniently renamed PF-06447475, and their preclinical data on this drug looks interesting:

Title: Leucine-rich Repeat Kinase 2 (LRRK2) Pharmacological Inhibition Abates α-Synuclein Gene-induced Neurodegeneration
Authors: Daher JP, Abdelmotilib HA, Hu X, Volpicelli-Daley LA, Moehle MS, Fraser KB, Needle E, Chen Y, Steyn SJ, Galatsis P, Hirst WD, West AB.
Journal: J Biol Chem. 2015 Aug 7;290(32):19433-44.
PMID: 26078453 (This article is OPEN ACCESS if you would like to read it)
In this study, the researchers gave either a placebo or PF-06447475 to G2019S-LRRK2 rats that were over producing human alpha synuclein in the dopamine neurons of the substantia nigra. The placebo-treated G2019S-LRRK2 rats had high levels of inflammation and cell loss in the substantia nigra. This neurodegeneration and inflammation was significantly reduced, however, in the PF-06447475 treated rats. Importantly, they could not detect adverse pathological damage in the lung, kidney, or liver of rats treated with PF-06447475 (I’ll come back to this in a moment).
These results led the researchers to question whether LRRK2 inhibitors could reduce the level of alpha synuclein accumulation in Parkinson’s disease, which resulted in this follow up research report last year:

Title: G2019S-LRRK2 Expression Augments α-Synuclein Sequestration into Inclusions in Neurons.
Authors: Volpicelli-Daley LA, Abdelmotilib H, Liu Z, Stoyka L, Daher JP, Milnerwood AJ, Unni VK, Hirst WD, Yue Z, Zhao HT, Fraser K, Kennedy RE, West AB.
Journal: J Neurosci. 2016 Jul 13;36(28):7415-27.
PMID: 27413152 (This article is OPEN ACCESS if you would like to read it)
In this study, the investigators wanted to know whether there was any connection between alpha synuclein, LRRK2, and the formation of protein aggregates (e.g. Lewy bodies). They found that G2019S-LRRK2 protein, both in cell culture and rats, increases the recruitment of alpha synuclein into the protein aggregates. And this effect was specific to the mutant LRRK2 protein as normal LRRK2 did not give the same results. Next they treated the cell cultures with their LRRK2 inhibitor PF-06447475 and they found that it blocked the recruitment of alpha synuclein effect caused by the G2019S-LRRK2 protein.
These preclinical results sound encouraging. Why aren’t they rushing to the clinic?
Way up the top of this post I mentioned something about LRRK2 having lot of functions. And we are still learning about those functions.
Some LRRK2 inhibitors appear to cause any changes in normal dopamine function in the brain while others do not (Click here to read more on this). Selecting the better drugs in this regard is a straight forward process. But there have been other cases for concern with regards to other organs in the body.
For example, researchers found negative effects of their LRRK2 inhibitor in the lungs. Mice born with no LRRK2 have morphologic changes in the lungs and kidneys. When the researchers treated mice with their LRRK2 inhibitors, they saw no changes in lung or kidney function. But when they then tried the same drugs in primates they found normal kidney function, but significant toxicity issues in the lungs (Click here to read more about this).
Given the cross-species differences and wide range of functions LRRK2 has in different tissues, caution is required as we move towards the clinic with these drugs.

Caution is required. Source: Mysafetylabels
For those interested in reading more about the development of LRRK2 inhibitors, click here and here for two good recent reviews.
One last question: since G2019S-LRRK2 and APP are interacting and causing trouble, is there any association between G2019S-LRRK2 and Alzheimer’s disease?
That’s a good question.
And the answer is: No, there isn’t.
There have been numerous studies looking at this and they have all found no link between G2019S-LRRK2 and Alzheimer’s disease (Click here, here, here and here for examples of this research).
So what does it all mean? (Or is there still more?)
No, we’ll sum up here: Researchers have recently found that a protein associated with Parkinson’s disease, LRRK2 is interacting with a protein associated with Alzheimer’s. The consequences of this interaction may lead to cell death in cases where a specific mutation exists within the LRRK2 gene. Luckily for people affected by this mutation, treatment options may soon be available in the form of LRRK2 inhibitors.
I find it truly remarkable that it was only 13 years ago that mutations in the LRRK2 gene was associated increased risk of developing Parkinson’s disease (Click here and here for the original reports), and here we are discussing potential clinical trials. Knowing how much effort goes into research and the length of time required for some experiments to be done (for example the aged mice used in the report discussed in this post were almost 2 years old!), I marvel at how rapidly things are now progressing within the Parkinson’s disease field.
Major questions still remain before these inhibitors enter the clinic though, particularly with regards to safety. In addition, there are important questions for the Parkinson’s community, such as who could be treated with these LRRK2 inhibitors? Will they have efficacy beyond people with the LRRK2-G2019S mutation Parkinson’s disease? Only time will tell. I’m regularly looking for news of a LRRK2 inhibitor heading for the clinic and I’ll let you know when I hear of one.
The banner for today’s post was sourced from Youtube
I think this is an excellent review of the science behind LRRK2 and Parkinson’s. It is becoming increasingly clear that there is no clear differentiation between Parkinson’s and Alzheimer’s. It also apparent that APP has a role to play in both diseases and that the internal domain of APP – AICD is a key player. I have been involved in several research programs aimed at finding selective kinase inhibitors and one thing is clear ‘selective’ is a relative term. No ‘selective’ kinase inhibitor is as specific as the originators would claim due to the large number of kinases and the relative preservation of the active site. This observation has two consequences. Firstly, there is a great risk of off-target toxicity which may not be such an issue for kinase inhibitors aimed at cancers but certainly is for chronic diseases such as PD and AD. Secondly, no two LRRK2 inhibitors will have the same efficacy/toxicity profile.
An alternative approach is to target AICD itself. We are developing a compound which binds to AICD and inhibits its translocation to the nucleus. This compound has completed a Phase 2 trial in Alzheimer’s with very promising results. We are now exploring its potential for both LRRK2 G2019S and idiopathic PD.
LikeLike
Hi Dr Hobden,
Thank you for your very interesting comment. While of lot of exciting research is being conducted on the LRRK2 inhibitors, I too am rather worried about their specificity/off-target issues, particularly what effect they may have on LRRK1 (given the level of homology between the two). As you suggest, a more targeted approach would be better. We will be very interested to hear about your drug in due course.
Kind regards,
Simon
LikeLike