|
# # # # Each year in September, The Cure Parkinson’s Trust and Van Andel Institute hold the international Linked Clinical Trials (iLCT) meeting. This is a drug-repurposing initiative focused on disease modification in Parkinson’s. For two days the iLCT committee discuss and debate the virtues of 20+ molecules to decide which should be prioritised for clinical evaluation. Due to the current COVID-19 situation, the 2020 iLCT meeting was held virtually. In today’s post, we will discuss what the iLCT program is and provide an overview of what happened at the 2020 meeting. # # # # |
The top line results of the PD-STAT clinical trial evaluating the cholesterol-reducing drug simvastatin in Parkinson’s were recently announced (Click here to read more about this). Preclinical data had suggested that this agent displayed neuroprotective properties in models of Parkinson’s, and given its long history of clinical use and agreeable safety profile, simvastatin seemed like an ideal candidate for repurposing to Parkinson’s.
A large Phase II clinical trial was set up and conducted across nation-wide network of 23 hospitals in the UK. It recruited over 230 brave individuals to be treated with the drug for 2 years and undergo regular clinical assessments.
The results of the study found that the treatment has had no impact on slowing the progression of Parkinson’s (Click here to read more about this).
That’s disappointing. What happens next?
Disappointing as the result is, the findings of the study provide us with a definitive answer, allowing us to move forward with testing other drugs of interest.
Simvastatin was a drug that was prioritised by the international Linked Clinical Trials programme, and while this agent might not have shown any beneficial efforts in Parkinson’s the good news is that there are lots of other drugs that have been prioritised by the international Linked Clinical Trials programme and they are now being clinically tested.
What is the international Linked Clinical Trials programme?
The Linked Clinical Trials (LCT) initiative was set up 9 years ago by The Cure Parkinson’s Trust with the goal of rapidly repurposing drugs that may have disease modifying potential for Parkinson’s.
What is meant by repurposing?
Drug repurposing (repositioning, reprofiling or re-tasking) is a strategy of identifying novel uses for clinically approved (or experimental) drugs that fall outside the scope of the original medical indication.
An example of this is Viagra.
 Source: RioTimes
Source: RioTimes
It was riginally developed as an anti-hypertensive medication, and was hugely more successful in the treatment of erectile dysfunction.
Another example is Amantadine.
 Source: Dailymed
Source: Dailymed
Originally developed as a prophylactic treatment against influenza, an incidental observation in 1969 led to it being repurposed for Parkinson’s.
A woman with PD was administered amantadine to treat an influenza infection. She reported that her Parkinson’s symptoms improved while on amantadine, and they worsened after she finished the treatment. This observation resulted in clinical trials, which ultimately led to a new treatment option for the Parkinson’s community.
The drug repurposing strategy has been adopted and applied by many organisations because it allows for the by-passing of large parts of the drug discovery process, saving time and resources in getting new treatments to the clinic.
 Source: Austinpublishinggroup
Source: Austinpublishinggroup
By repurposing a clinically approved drug – for which we may know a great deal about already in terms of safety, tolerability and dose range – we can skip large parts of the clinical trial process and jump straight to testing the drug in our population of interest (in this case people with Parkinson’s).
In addition to repurposing drugs, the LCT initiative also seeks to catalyse the clinical testing of new experimental therapies in Parkinson’s. A biotech company developing a new molecule may not necessarily be targeting Parkinson’s as their primary indication (disease of interest). But if the biological target of the drug is Parkinson’s-relevant, why should the PD community wait for the drug to be approved by health regulators before it is tested in PD? The LCT initiative provides a potential opportunity to start testing that drug in Parkinson’s earlier.
How did the LCT programme start?
The tale begins not with neurology, but cardiology.
Specifically, a cardiologist. His name is Dr Richard Wyse:
 Dr Richard Wyse with an LCT dossier. Source: Trendsmap
Dr Richard Wyse with an LCT dossier. Source: Trendsmap
Richard is the director of research at The Cure Parkinson’s Trust and he has been the driving force behind the development and progress of the LCT programme. It is is basically his brain child. And it all began when Richard met the legandary Tom Isaacs (Parkinson’s advocate and one of the co-founders of CPT) at a scientific conference shortly after the Trust was set up. They got talking and over a period of time Tom eventually talked Richard into joining the fledgling organisation.
 The man, the myth, the legend: Tom Isaacs
The man, the myth, the legend: Tom Isaacs
Richard joined the Cure Parkinson’s Trust in 2007 and he immediately began working on preparations for both the Exenatide and GDNF clinical trial programmes for Parkinson’s (Click here and here to read previous SoPD posts about those drugs).
In 2009, Tom was becoming increasingly frustated with the lack of research around the world that was focused on actually “curing” Parkinson’s. He did not want a new iteration on an old symptomatic treatment, he was looking for treatments that would actually change the trajectory of the condition. And in their conversations at CPT HQ, Tom kept asking Richard “What can we do to accelerate things?”
This question resonated with Richard and gradually the seed of an idea came to life. And that idea gave rise to the Linked Clinical Trials initiative.
In essence, the initiative involves a committee of international Parkinson’s experts meeting annually to evaluate a number of compounds for their disease modifying potential in Parkinson’s, and the committee would prioritise which one should be clinically evaluated. Once prioritised CPT would be mandated to getting that drug into a clinical trial for disease modification in Parkinson’s.
Here is a video of Dr Richard Wyse providing an overview of the LCT intiative:
In 2010, the Cure Parkinson’s Trust began building the committee of Parkinson’s experts for the Linked Clinical Trials initiative, and they asked Prof Patrik Brundin who is the director of the Center for Neurodegenerative Science and Jay Van Andel Endowed Chair at the Van Andel Institute (Michigan) to be the chairperson of the LCT committee.
Prof Brundin is a world-renowned scientist in the field of Parkinson’s research. He has more than 30 years of experience (and over 350 research articles) investigating Parkinson’s pathology and experimental therapeutic approaches for the condition. He has also played an instrumental role in the development of the LCT programme, which has now been rebranded as the international Linked Clinical Trials (iLCT) initiative.
 Prof Patrik Brundin. Source: Mlive
Prof Patrik Brundin. Source: Mlive
Here is Prof Brundin discussing the LCT initiative:
The Cure Parkinson’s Trust and the Van Andel Institute formed a partnership to take the iLCT programme forward, and with tremendous support from both groups there has been no looking back:
 Prof Brundin & Tom Isaacs signing the partnership agreement. Source: CPT
Prof Brundin & Tom Isaacs signing the partnership agreement. Source: CPT
Current members of the LCT committee include some of the best Parkinson’s researchers in the world:
- Prof Roger Barker (University of Cambridge, UK)
- Prof Flint Beal (Cornell University, USA)
- Dr Camille Carroll (Plymouth University, UK)
- Dr Mark Cookson (NIH, USA)
- Prof Ted Dawson (Johns Hopkins University, USA)
- Prof David Devos (University of Lille Nord de France)
- Prof Jeffrey Conn (Vanderbilt University, USA)
- Prof Howard Federoff (University California at Irvine, USA)
- Dr Brian Fiske (The Michael J Fox Foundation)
- Prof Tom Folytnie (University College, London, UK)
- Prof Karl Kieburtz (University of Rochester, USA)
- Prof Dimitri Krainc (Northwestern University Feinberg School of Medicine, USA)
- Prof Andrew Lees (University College London, U.K)
- Prof Mark Mattson (Johns Hopkins University, USA)
- Prof Michael Schwarzschild (Harvard Medical School, USA)
- Prof David Simon (Harvard Medical School, USA)
- Prof David Sulzer (Columbia University, USA)
- Prof Caroline Tanner (University of California, San Francisco, USA)
- Prof John Trojanowski (University of Pennsylvania, USA)
- Prof Tim Greenamyre (University of Pittsburgh, USA)
The first LCT meeting was held in 2012, and a report on the initiative and that meeting was published early the next year.
This is the report:
 Title: Linked clinical trials–the development of new clinical learning studies in Parkinson’s disease using screening of multiple prospective new treatments.
Title: Linked clinical trials–the development of new clinical learning studies in Parkinson’s disease using screening of multiple prospective new treatments.
Authors: Brundin P, Barker RA, Conn PJ, Dawson TM, Kieburtz K, Lees AJ, Schwarzschild MA, Tanner CM, Isaacs T, Duffen J, Matthews H, Wyse RK.
Journal: J Parkinsons Dis. 2013 Jan 1;3(3):231-9. doi: 10.3233/JPD-139000. Review.
PMID: 24018336 (This report is OPEN ACCESS if you would like to read it)
In this report, the committee discussed some of the drugs that were evaluated that year. Since that first meeting, however, the committee has decided not to put the results of subsequent meetings in the public domain for two important reasons:
- Confidentiality – some of the information being discussed at the meeting is of a confidential and (in some cases) commercially sensative nature, and
- Safety – very little is known about what might be the right dose to be effective for each compound (if a right dose even exists). And even if an effective dose can be ‘estimated’, even less may be known about the safety of that particular dose. Such safety testing needs to be carried out at hospital research centres.
Thus, the results of the meetings are now not disclosed.
So how are the drugs selected?
Ok, so this is my favourite part of the whole thing.
In fact, the process of identifying drugs/treatments of potential interest for the LCT meetings has very quickly become something of an obsession for yours truly.
And I’m talking “call the divorce lawyers”-kind of obsession.
I can regularly be found sitting in my arm chair at home at 3 am snooping around the internet looking for clues or connections between Parkinson’s and a particular drug. It is like an academic treasure hunt or a jigsaw puzzle – but one that I am utterly consumed by.
 Finding the pieces. Source: Medium
Finding the pieces. Source: Medium
The research team at the Cure Parkinson’s Trust maintains a constant vigil on the neurodegenerative research being published, but we will also often go walking off into left field seeking novel ideas from other medical conditions (such as oncology). We also regularly ask for and discuss new ideas with LCT committee members.
Once we find something interesting, we will approach researchers and talk to biotech companies about compounds that target a particular biological pathway of interest.
Many of these biotech companies are not focused on Parkinson’s or neurodegeneration (or even considering it as a possible indication for their drug). They may have a drug that is being clinically tested for a liver condition, and its disease modifying potential in a brain-related condition is definitely not part of their business plan or even on their radar.
On other occasions (as we discussed above), a particular drug/molecule of interest may not have any intellectual property attached to it and there will be no company to talk to.
Each case is different.
When an interesting drug is identified, the CPT research team will begin investigating everything that is known about the basic characteristics of the drug and what it does in the body. For example, in most cases it is important to know how well:
- the drug can access the brain which is surrounded by a protective membrane that limits most drugs from entering. This is called ‘brain penetrance‘.
- the drug is absorbed by the body. This is called ‘bioavailibility‘.
- the drug lasts in the body. This is called ‘half-life‘.
- the action of the drug can be measured. This is called ‘target engagement‘.
Most critically, however, the committee will be rightly focused on the safety and tolerability of the compounds being discussed, as they do not want to prioritise a treatment that could potentially do harm or reduce the well being/quality of life of the individuals being tested. So drugs that have been tested in humans before and have a well known clinical profile will have an advantage over others.
Once the CPT research team has all of this information, they will then decide to write a dossier for the drug to present to the iLCT committee for evaluation.
What goes into the dossier?
Everything.
Everything that we know about the drug and also the argument/rationale for testing it in Parkinson’s for disease modification. Here is a video of someone taller, but less attractive than Richard showing you what the collection of dossiers looks like:
(Face for radio, voice for silent film)
And what happens at the meeting itself?
So in any normal year, the meeting is a 2 day event held at the Van Andel Institute in Grand Rapids (Michigan).
 The Van Andel Institute. Source: VAI
The Van Andel Institute. Source: VAI
It is a closed door, invite-only meeting, but serious efforts are made to have representatives from all of the major Parkinson’s organisations in the room. For example, the Michael J Fox foundation, the Silverstein Foundation, Parkinson’s UK, and Shake it Up Australia all have representatives at the meeting. Government and regulatory organisations (such as the NIH) are also invited. But critically a group of patient advocates are also in attendance.
Thus, all of the major stakeholders are present and every voice is listened to.
 Source: CPT
Source: CPT
The iLCT committee sit at the front of the room in a horse shoe arrangement, and individuals members will present a dossier for discussion. Each dossier is debated for about 30 minutes. The other attendees in the room are regularly asked for their opinion/thoughts, and there is a very dynamic discussion before the committee members are asked to vote on each dossier.
At the end of the day, the dossiers will be ranked according to their scores and 3-5 drugs will be prioritised for clinical testing.
The job of the iLCT committee is primarily drug selection, but given the expertise sitting around the table there is usually some discussion about what potential clinical trial design might look like and which subtypes of Parkinson’s could be investigated for particular drugs.
Interesting. So what has been the outcome of this process?
The iLCT programme has given rise to 7 completed clinical trials and 15 drugs currently being clinically tested in 16 clinical trials at research centres around the world. One of the ongoing clinical trials is the Phase III Exenatide study that has just started here in the UK.
Can you give an example of a drug that was prioritised?
Exenatide (or Byrureon) – a diabetes treatment is a good example. It was the first drug to be prioritised in the LCT process.
There was preclinical data supporting a neuroprotective effect in models of Parkinson’s and a great deal was known about the clinical profile of the drug. A Phase I clinical trial of Exenatide in Parkinson’s had also been conducted.
The results of the trial were published in 2013:

Title: Exenatide and the treatment of patients with Parkinson’s disease.
Authors: Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, Limousin P, Foltynie T.
Journal: J Clin Invest. 2013 Jun;123(6):2730-6.
PMID: 23728174 (This study is OPEN ACCESS if you would like to read it)
In this study, the researchers gave exenatide (the Byetta formulation, which is injected twice per day) to a group of 21 people with moderate Parkinson’s and evaluated their progress over a 14 month period. They compared those participants to 24 additional subjects with Parkinson’s who acted as control (they received no treatment beyond their normal PD medication). Exenatide was found to be well tolerated by the participants.
Importantly, the exenatide-treated subjects demonstrated improvements in their Parkinson’s movement symptoms (as measured by the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (or MDS-UPDRS)), while the control patients continued to decline.
Interestingly, in a two year follow up study – which was conducted 12 months after the subjects stopped receiving exenatide – the researchers found that participants previously exposed to exenatide demonstrated a significant improvement (based on a blind assessment) in their motor features when compared to the control subjects involved in the study (Click here to read more about this).
It is important to remember, however, that this trial was an ‘open-label study’ – that is to say, the participants knew that they were receiving the exenatide treatment so there is the possibility of a placebo effect explaining the improvements. And this necessitated the testing of the efficacy of exenatide in a Phase II double blind clinical trial.
And the results of that trial were published last August (2017):
 Title: Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial
Title: Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial
Authors: Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T
Journal: Lancet 2017 Aug 3. pii: S0140-6736(17)31585-4.
PMID: 28781108 (This report is OPEN ACCESS if you would like to read it)
In this study, the investigators recruited 62 people with Parkinson’s (average time since diagnosis was approximately 6 years) and they randomly assigned them to one of two groups, exenatide (the Bydureon formulation which is injected once per week) or placebo (32 and 30 people, respectively). The treatment was given for 48 weeks (in addition to their usual medication) and then the participants were followed for another 12-weeks without exenatide (or placebo) in a ‘washout period’.
In this trial everyone was “blinded” – meaning that both the investigators and the participants did not know who was receiving the exenatide or placebo treatments. This is referred to as a double-blind clinical trial and is considered the gold standard for testing the efficacy of a new drug.
The researchers found a statistically significant difference in the motor scores of the exenatide-treated group verses the placebo group (p=0·0318). As the placebo group continued to have an increasing (worsening) motor score over time, the exenatide-treated group demonstrated improvements, which remarkably remained after the treatment had been stopped for 3 months (weeks 48-60 on the graph below).
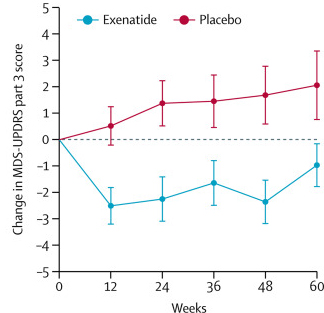
Reduction in motor scores in Exenatide group. Source: Lancet
Brain imaging (DaTscan) also suggested a trend towards reduced rate of decline in the exenatide-treated group when compared with the placebo group. Interestingly, the researchers found no significant differences between the exenatide and placebo groups in scores of cognitive ability or depression – suggesting that the positive effect of exenatide may be specific to the dopamine or motor regions of the brain. It should be noted here that the Phase II clinical trial for Exenatide was also supported by the Michael J Fox Foundation.
A Phase III clinical trial for exenatide has recently been initiated to determine if the drug is having a long term impact on Parkinson’s. 200 people will be recruited at 6 UK research centres and followed over 2 years (Click here to read more about this).
But in addition to exenatide, there are a LOT of other iLCT clinical trials underway.
And at the 2020 iLCT meeting, the whole event started with an overview of the ongoing iLCT studies.
So what happened at the 2020 iLCT meeting?
Well the first thing to note this year was that the event was virtual and held over 4 half days rather than 2 full days. The first day was dedicated to updates, which included news that:
- The Ambroxol trial results have been published and preparations for a larger study are underway (Click here to learn more about this).
- The Exenatide/Bydureon Phase III trial has started (Click here to learn more about this).
- The Simvastatin trial has finished and the initial results indicate that the drug has no disease modifying effect on PD (Click here to learn more about this).
- The Liraglutide trial in California will be finishing next year (2021 – click here to learn more about this).
- The Lixisenatide Study in France will be finishing next year (2021 – click here to learn more about this).
- The UDCA trial in the UK is nearing completion and the results will be available next year (2021 – Click here to learn more about this).
- The first multi-arm clinical trial of the Australian Parkinson’s Mission is underway (Click here to read more about this).
 And a lot of other news/updates was shared with the committee members.
And a lot of other news/updates was shared with the committee members.
What about the other days of the meeting?
During the other days of the meeting, the dossiers of candidate drugs were discussed.
At the 2019 iLCT meeting, the committee members specifically asked for drugs targetting novel mechanisms of action. The committee wanted new targets and it is important for the Parkinson’s research community to focus on this. We have numerous ongoing clinical trial programs that are testing different types of alpha synuclein targetting therapies or anti-inflammatory approaches. While we wait for those trials to read out, we need to be exploring new pathways.
So for the last 12 months the research department at The Cure Parkinson’s Trust has been on the hunt for ‘novelty’. Agents whose mechanisms of action have not been clinically explored in Parkinson’s, but that are ready for clinical evaluation.
No easy task.
At the meeting the committee were presented with a variety of dossiers exploring such targets as a very specific anti-bacterial approach, an aldehyde reducing agent, and a specific chemokine receptor inhibitor.
Of all the dossiers presented, just three were prioritised for clinical evaluation, and CPT (with support from their funding partners) will now move those three agents into clinical trials.
Sounds amazing. But why is the meeting only once per year?
The answer to this question is a combination of the availability of the LCT committee members (who are all very busy running major research programmes as well as maintaining clinical work) and a limited amount of resources (it takes time and money getting clinical trials started – currently, one iLCT meeting per year is enough).
So what does it all mean?
It doesn’t need to be said, but there is a desperate need for novel therapies that will slow, stop or reverse Parkinson’s.
And a great deal of Parkinson’s research that is focused on investigating new treatments for limiting the progression of this condition (for a good example of a novel experimental therapy see the recent SoPD post on the Sigma-1 receptor agonist ANAVEX2-73/blarcamesine – click here to read that post). Thus, while the PD-STAT results are disappointing, they are not the only approach being evaluated for disease modification in PD and it is encouraging that so many alternative therapies are also being tested.
The international Linked Clinical Trials initiative is the fastest way that the Cure Parkinson’s Trust and Van Andel Institute can think of to get novel therapies to the Parkinson’s community.
And as we now seek to get three more iLCT prioritised drugs into clinical trial, the research team at CPT are already sniffing out candidate drugs for the 2021 LCT meeting (at present I have 4 compounds of interest and the next meeting is still 11 months away!).
 All of the material on this website is licensed under a
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
EDITOR’S NOTE – The author of this post is an employee of the Cure Parkinson’s Trust and utterly obsessed with the Linked Clinical Trials process, so he might be a little bit biased in his views on the topic. The trust has not requested the production of this post, but the author considered it interesting and important to share with the Parkinson’s community.
In addition, the information provided by the SoPD website is for educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from CPT.




Research that is specifically focused on slowing or reversing progression is desperately needed. All of the palliative therapies presently available work less well as the disease progresses. Ultimately, there is no substitute for as close to a normal population of functioning dopamine neurons as one can retain.
But I have a question: Why is every study of this effort focused on testing a single drug? If devising a therapy is based on a model of the disease process, then why not identify multiple ways to interfere with that process, and then target as many as possible, and test the resulting combination therapy? That is, why are we not testing *protocols* for treatment, then, rather than individual drugs?
And why are so many (all?) of the candidates prescription drugs, rather than including some non-prescription candidates?
It seems to me that the habit of testing individual drugs has emerged from the for-profit pharmaceutical industry, which is interested in profiting from individual products, and which must have individual products certified for use. But given that this effort is dealing with drugs that have already been approved, why should it be limited to testing monotherapies?
Now, one might answer that testing multiple drugs at once does not allow us to determine which drug was responsible for a given improvement. But what if the best therapy is a combination therapy, involving a set of agents that individually may have little or no effect? By testing individual substances one at a time, one might never discover that combination effect.
I am thinking specifically of the autoimmune feedback loop that seems to be central to the destruction of dopaminergic neurons in the nigrostriatal area.
A feedback loop is very sensitive to thresholds. In an auto feedback loop, for example, you might turn down the amplifier, or you might move the microphone further away from the speaker, or you might employ a unidirectional microphone, or place a hood around the speaker and the microphone. It may be that stopping the feedback will only occur when you employ more than one of these approaches. With any one method, there might still be a howl that drowns out any nuances introduced by that approach.
So in Parkinson’s autoimmune feedback, we might interfere by the use of substances that block receptors on microglia, so that they are not stimulated to attack by the neuromelanin and aggregated alpha-synuclein. More than one such blocking substances may be required to prevent microglia from shifting in the the M1 attack state. And we might employ other substances that improve mitochondrial function, so that neurons are better able to resist the attacks leveled at them via cytokines released by the microglia. And we might employ antioxidants that also mitigate the actions of such cytokines. And we might employ agents that interfere with the aggregation of alpha-synuclein. And so on–interfering at every available point of the cycle that drives autoimmune inflammation.
So, why not test a protocol involving more than one substance?
LikeLike
Hi Lou,
Thanks for the comment and interesting questions. Regarding mono-therapies, the approach clinicians have taken thus far has been that we need an agent to give us a positive, measurable signal before we can start bolting on additional agents, and even then it will be a very delicate shift. Safety is always the primary concern – no one wants to be responsible for making another individual’s sitation worse.
Ultimately we are heading for a cancer-like “treatment cocktail” encompassing different therapies in order to better personalise treatment to individuals circumstances, but at present we need molecules that show signs of shifting the needle before we can start combinine them. It is pointless to attempt multiple therapies if they are both barking up the wrong tree (eg an iron chelator-GLP-1 agonist combo would probably have no impact if PD is caused by a viral infection). At present, in the absence of a clear mechanism of action for PD (and biomarkers to assess target engagement), moving forward with combination therapies would be reckless.
I certainly agree on the need for multi-treatment approaches. I have often worried that boosting one cellular function (such as glycolysis, which might be good for PD) may have undesirable side effects (perhaps increased oxidative stress?) that might cancel out any potential benefit. So the justification for multi-treatment approaches is reasonable.
We will, however, need to be very careful with that first combination as there may be molecules that are contraindicated. If GLP-1 agonists provide the first signal (and I’m not speculating that they will be, I am simply using them as an example here), then the first dual therapies might come in the form of GLP-1 & GIP dual agonists (like Tirzepatide/LY3298176 being developed by Lilly). This would be an iterative step in the combination direction. And then if immunotherapy is then found to be effective in slowing PD, one could envisage a GLP-1 agonist/immunotherapy combination (I am simply using these as examples here).
Having said all of this, there are already combination efforts underway in other neurodegenerative conditions (such as Amylyx’s AMX0035 (a sodium phenylbutyrate–TUCDA combo) which recently had good results in ALS: https://www.nejm.org/doi/full/10.1056/NEJMoa1916945 & https://onlinelibrary.wiley.com/doi/10.1002/mus.27091).
Oh, and not all of the iLCT candidates are prescription drugs. The iLCT committee has previously prioritised non-prescription molecules.
Kind regards,
Simon
LikeLike
“Auto feedback” above should read “audio feedback.” This error was due to “audio correct.” 🙂
LikeLike
Thank you for this interesting blog, Simon. And thank you even more for looking obsessively into the early hours for possible LCT drugs. Very good to know in these grim times that someone is quietly beavering away to make things less grim.
Tried your dressing gown pocket? That’s normally where I find stuff.
Have you tried your dressing
LikeLike
Hi Jelly,
Thanks for your comment. Glad you liked the post.
No dressing gown. Think Spiderman onesie.
🙂
Simon
LikeLike
I strongly second Lou T’s comments. The focus on mechanisms identified from pre-clinical research is reasonable but limiting, since we know that PD is multifactorial. The human brain is a complex system with compensatory mechanisms. If we only attack a single mechanism, are we really attacking enough of the mechanisms to demonstrate clinical benefit? On a side note, I am wondering whether patient advocates were present for the virtual meeting this year.
LikeLike
Hi Karen,
Thanks for your comment.
The number of participants in the iLCT meeting this year was less than previous years due to the virtual format of the meeting and the increased number of dossiers that were discussed under NDAs. PD research advocates were involved in the meeting, but there were less this year (as with all of the guests) due to the unique circumstances.
I hope your leg is healing up ok.
Simon
LikeLike
Hi Simon,
you describe how amantadine was discovered as a treatment for Parkinson’s: “A woman with PD was administered amantadine to treat an influenza infection. She reported that her Parkinson’s symptoms improved while on amantadine, and they worsened after she finished the treatment.”
I (PwP) can report the same for brivudine, which I took last spring to treat shingles. I know that the package leaflet states that “Post-marketing experience indicates a possible interaction of brivudin with anti-Parkinson dopaminergic drugs, that may facilitate the onset of chorea (abnormal, involuntary, dance-like movements, especially of arms, legs and face).” In my case, however, brivudine led to a dramatic and unexpected improvement in my motor skills, without any “chorea” occurring. So I am now looking for a Parkinson’s researcher who might be interested in this observation. Maybe there is something to be discovered. I would be happy to provide more details.
zz
LikeLike
I too totally support the comments by the previous people about the need to break or reduce as many parts of the destructive cycle, and the importance of testing which non-prescriptive substances and actions such as exercise best complement each other and support whatever prescription drug is involved.
Being realistic about a process that has probably taken many years before the straw broke the camels back, an overnight cure is unlikely, but slowing deterioration and preserving as many of those neurons as possible is a critical first step. I wonder how long participants in “failed” trials are followed up afterwards? Even if there was no obvious improvement they may actually have reduced the speed of decline. Only follow-up after 5 or 10 years would give an indication.
I am certainly convinced from observations that a high exercise regime and vitamin D are two components and nicotinamide riboside helps with lack of mental and physical energy.
LikeLike
Thanks for great writeup, Simon, and for the work of CPT’s research team. I think your “(Face for radio, voice for silent film)” is backwards, though. Must be due to auto-correct (or “audio-correct” per Lou T above)…lol.
LikeLike
The USS Scorpion was lost at sea in 1968. The method used to locate the sunken sub was to gather input from a diverse group of experts: sub commanders, sonar experts, engineers, salvage experts etc. All the inputs of last known position and potential causes were weighted, assigned probably, analyzed, revised until a probability map of expected location of the sub was generated and maintained as new info and theories arrived. Apparently the experts involved were even betting bottles of scotch as to the final resting place of the Scorpion.
The LCT process feels fever much an analogue of this proven process.
Coincidentally, the man credited with pioneering what is now known as Bayesian search theory, John Craven, had Parkinson’s.
LikeLike