|
At the end of each month the SoPD writes a post which provides an overview of some of the major pieces of Parkinson’s-related research that were made available during April 2020. The post is divided into seven parts based on the type of research:
|
So, what happened during April 2020?
In world news:
April 2nd – Billionaire Amazon founder Jeff Bezos donated $100 million to Feeding America, a nonprofit that helps food banks feed families in need (Source).
April 6th – In March, billionaire Jack Ma donated 1.1 million COVID-19 testing kits, 6 million masks and 60,000 medical use protective suits/face shields to Africa. On this date, he sent a second shipment (including 500 ventilators – source).
April 7th – Billionaire founder of Twitter, Jack Dorsey, announced he was donating $1billion (28% of his net wealth) to help fund COVID-19 relief (source).
 (For more on how billionaires are supporting the COVID-19 effort – click here)
(For more on how billionaires are supporting the COVID-19 effort – click here)
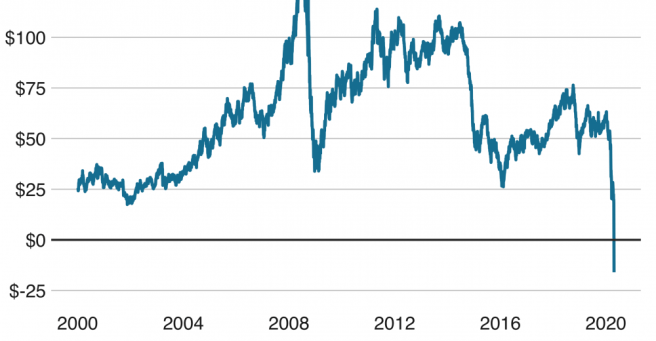
April 20th – Oil prices reached a record low (falling into negative values) due to the ongoing coronavirus pandemic and the Russia–Saudi Arabia oil price war
April 30th – Captain Tom Moore – a war veteran who has raised over £32.79 million for the NHS by completing 100 laps (25m/27yd) of his garden in 10 days – celebrated his 100th birthday
In the world of Parkinson’s research, a great deal of new research and news was reported:
In April 2020, there were 976 research articles added to the Pubmed website with the tag word “Parkinson’s” attached (3340 for all of 2020 so far). In addition, there was a wave to news reports regarding various other bits of Parkinson’s research activity (clinical trials, etc).