|
In 2013, a biotech company called Ceregene reported disappointing results from their experimental gene therapy clinical trial for Parkinson’s. The data from the study suggested that the therapy had no clinical effect on the progression of Parkinson’s (Click here to read the press release). Today, however, researchers associated with that biotech company have published a new report that suggests that the treatment had beneficial effects in the brain, but not enough of it was delivered. The treatment was a gene therapy approach (which involves using DNA rather than drugs to treat medical conditions), and it involved a protein called neurturin. In today’s post, we will discuss what neurturin is, we will review what this new study found, and consider what the implications could be for future gene therapy trials in Parkinson’s.
|
 Source: Medium
Source: Medium
Reanalysing clinical trial data (called post-hoc analysis) provides a very useful way of generating new hypotheses even if the initial study did not reach its primary endpoint (that is to say the study did not demonstrate a successful outcome. Post-hoc analysis must be handled carefully, as the findings of such investigations can be viewed as selective ‘cherry picking’ of interesting outcomes. They will need to be tested to determine if they are real effects.
Even more important than post-hoc analysis, however, is following up participants who took part in a trial to see if there were any long-term benefits from the treatment. I often wonder how much important data is lost after a clinical trial simply becomes there is no long term follow up and study investigators lose track of participants as they drift away.
Precious nuggets of information can be gained from long-term analysis. And this week we saw a really interesting example of this.
Here is the research report:
 Title: Long-term post-mortem studies following neurturin gene therapy in patients with advanced Parkinson’s disease.
Title: Long-term post-mortem studies following neurturin gene therapy in patients with advanced Parkinson’s disease.
Authors: Chu Y, Bartus RT, Manfredsson FP, Olanow CW, Kordower JH.
Journal: Brain. 2020 Mar 1;143(3):960-975.
PMID: 32203581 (This report is OPEN ACCESS if you wouldl like to read it)
In this study, the researchers were looking at postmortem brain sections from 2 participants who took part in a clinical trial investigating a treatment called neurturin.
What is neurturin?
Neurturin is a neurotrophic factor.
What is a neurotrophic factor?
Neurotrophic factors (neurotrophic = Greek: neuron – nerve; trophikós – pertaining to food/to feed) are proteins that nurture neurons and support their growth. There are many types of neurotrophic factors, some having more beneficial effects on certain types of neurons and not other.
 Source: Helsinki
Source: Helsinki
Glial cell line-derived neurotrophic factor (or GDNF) is a neurotrophic factor that we have discussed many times on this website (Click here to read a previous SoPD post on GDNF).
Neurturin is a member of the same neurotrophic factor family as GDNF (and there are two other family members – persephin, and artemin – sounds like the Three Musketeers!). All of these family members have demonstrated positive effects on dying dopamine neurons (the vulnerable population of neurons in Parkinson’s).
The positive/neuroprotective effect of these neurotrophic factors works via a series of ‘receptors’ on the outer surface of cells. Receptors are molecules sitting on the surface of cells, waiting for specific proteins to come along and bind to them. By binding to them, the receptor becomes activated.
There are receptors that are specific for each of these GDNF family members, and when they bind to their respective receptor, they activate another surface protein called RET.
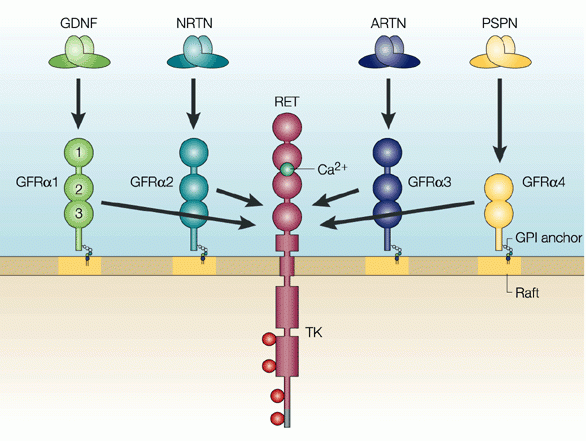 The GDNF family photo. Source: Nature
The GDNF family photo. Source: Nature
What is RET?
Ret proto-oncogene (or RET) is a receptor tyrosine kinase, that is a cell-surface molecule that initiates signals inside the cell after surface receptors are bound. The activation of RET results in cell growth and survival.
So neurturin has been tested in models of Parkinson’s?
Yes it has. The first report was is 1998: Title: Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons.
Title: Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons.
Authors: Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, Kirik D, Moffat B, Simmons L, Johnson E Jr, Milbrandt J, Rosenthal A, Bjorklund A, Vandlen RA, Hynes MA, Phillips HS.
Journal: J Neurosci. 1998 Jul 1;18(13):4929-37.
PMID: 9634558 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers reported that neurturin supported the survival of dopamine neurons in cell culture. But importantly, the protein had a neuroprotective effect when administered to a neurotoxin rodent model of Parkinson’s (at a similar potency to GDNF).
Other research groups have reported similar positive effect (for examples, click here and here to read more).
|
RECAP #1: Neurturin is a neurotrophic factor – this is a naturally occuring protein that nurtures and supports neurons. It belongs to the same family of proteins as GDNF. Neurturin has been shown to have neuroprotective properties in models of Parkinson’s.
|
Awesome, where can I buy some of this neurturin stuff from?
The problem with neurturin is that this protein does not cross the blood brain barrier very well. The blood brain barrier is a protective membrane that covers the brain and limits the types of molecules that can enter.
Due to this feature of the protein, much of the research in neurturin in Parkinson’s has focused on direct delivery of the protein into the brain or on gene therapy approaches.
What are “gene therapy approaches”?
Gene therapy is an experimental treatment approach that involves treating medical conditions with DNA rather than drugs.
Gene therapy basically involves introducing a new piece of DNA or replacing a faulty piece of DNA within a population of cells. DNA, as you may remember from high school science class provides the instructions for making proteins in the a cell and these proteins are the bits that actually do stuff.
 Gene therapy. Source: yourgenome
Gene therapy. Source: yourgenome
By introducing a new piece of DNA into a cell, the cell can start to produce a functioning protein (from that functional DNA) that it may not normally produce. In some diseases, a cell may normally produce a particular protein, but because the genetic instructions in the DNA (a section of the DNA called a gene) for that protein have a small error (a genetic mutation), a non-functioning version of the protein is actually being produced. The introduction of the new correct (functioning) version of that piece of DNA (or gene) into a cell can start the production of a functional version of the protein.
Alternatively, a gene can be introduced into a cell which would cause the cell to produce a protein that that cell usually does not produce. This sort of approach is being used in gene therapy for cancer, where ‘suicide genes’ are being introduced into cancer cells. These cause the cancer cell to die, by initiate an auto-destruct sequence resulting in cell death (a process called apoptosis). Another approach the cancer field is using is introducing a gene into cancer cells that cause a protein to be produced on the surface of the cancer cell that attracts the attention of the immune system. This ‘marker gene’ causes the immune system to attack the cancer cell, resulting in the death of the cancer cell.
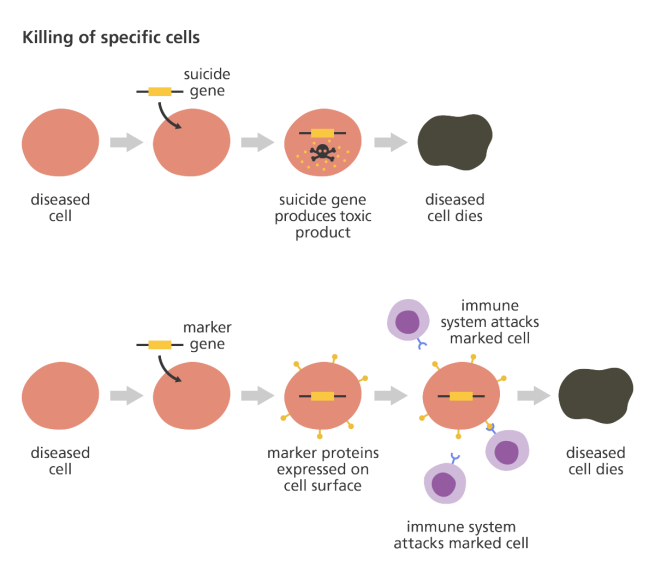 Source: yourgenome
Source: yourgenome
Taking this approach one step further, we can take sections of DNA that contain the genes involved with the production of a proteins that would be beneficial for the cell, such as GDNF or neurturin. By then injecting a virus with the DNA for neurturin into the brain, we can produce neurturin protein in any infected cells (it’s slightly more complicated than that, but you get the basic idea).

Gene therapy for Parkinson’s disease. Source: Wiki.Epfl
I’m sorry, but did you say viruses? I’m a bit sensative about viruses at the moment.
Yes, but if you remove the viral DNA from inside a virus and replace it with something useful, then a virus becomes a very useful biological delivery system. Far superior to anything we humans have devised thus far. Viruses are easy to produce and manipulate, and they can even be engineered to target specific cell types.
And these viruses have been engineered not to replicate. They deliver the proposed DNA and that is all.
|
RECAP #2: Neurturin has trouble accessing the brain when administered in the body. Gene therapy approaches have been employed to circumvent this issue. Gene therapy involves using DNA rather than drugs. Carefully engineered viruses can be used as biological delivery systems to get the DNA into the population of cells of interest. By infecting cells with neurturin DNA, those cells can be made to start producing neurturin protein.
|
Has this gene therapy approach ever been tested with neurturin?
Yes it has.
Almost immediately after the discovery of GDNF (and the other members of this neurotrophic family) was announced, researchers began trying to stick the DNA of of these proteins into empty viruses with the goal of infecting cells in the brain and causing them to produce the protein. The first successful demonstration of this feat with GDNF in a model of Parkinson’s was published in 1997:
 Title: Dopaminergic neurons protected from degeneration by GDNF gene therapy.
Title: Dopaminergic neurons protected from degeneration by GDNF gene therapy.
Authors: Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC.
Journal: Science. 1997 Feb 7;275(5301):838-41.
PMID: 9012352
In this study, the researchers inserted the DNA for GDNF into an adenovirus, and injected it into the part of the brain where the dopamine neurons reside (the substantia nigra). This treatment resulted in a 3 fold reduction in the loss of dopamine neurons 6 weeks after a neurotoxin (6-OHDA) was delivered (compared with no gene therapy or an empty virus control treatment).
And the first example of gene therapy neurturin rescuing a Parkinson’s was published in 2005 (Click here to read more about this).
But the type of virus used in gene therapy is important. Adenoviruses are known to cause immune responses in mammals. And this has caused researchers to shift to AAV viruses.
What are AAV viruses?
Adeno-associated viruses (or AAV) are a kind of virus that are popular with researchers because A.) they readily infect human and primate cells, and B.) they produce little (if any) immune response, and C.) they are non-pathogenic (they don’t cause any known diseases).
Given these characteristics, AAVs have been used in most of the gene therapy clinical trials thus far:
 AAV-based gene therapy clinical trials. Source: Wikipedia
AAV-based gene therapy clinical trials. Source: Wikipedia
They were originally discovered in the preparation of another type of virus, called an adenovirus (hence the name ‘Adeno-associated’). They were believed to simply be a contaminant of that preparation. Further research, however, revealed that AAVs belong to the Dependoparvo genus of viruses, which in turn belongs to the family Parvoviridae.
AAVs are single-stranded DNA viruses, and they are one of the smallest viruses (approximately 22 nm in diameter) with a non-enveloped capsid. The capsid is the shell surrounding the genetic material of the virus. Viruses are either enveloped or non-enveloped. “Enveloped” means that a second casing surrounds the capsid, providing further protection for the virus, while “non-enveloped” viruses have only the capsid.
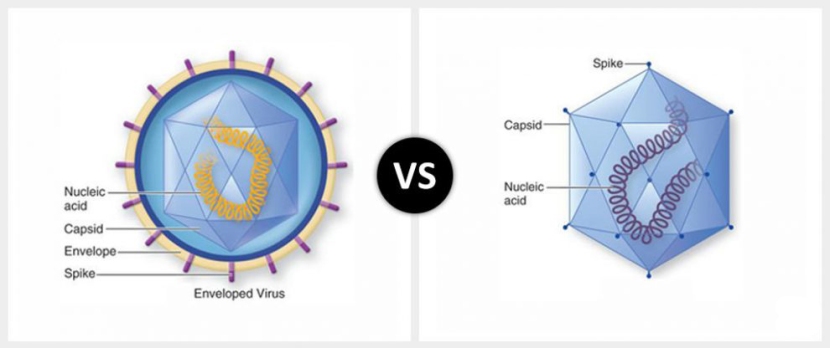
Enveloped (left) vs Non-enveloped (right) viruses. Source: Differencebtwn
Given the reduced amount of casing, non-enveloped viruses are generally more virulent (more infectious) than enveloped viruses (a good example of a non-enveloped virus is the influenza virus). Non-enveloped viruses do not survive outside of an organism for long though.

The AAV capsid. Source: Wikipedia
The first AAV virus containing neurturin was investigated in models of Parkinson’s in the early 2000s.
This was the first report of this gene therapy approach:
 Title: Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson’s disease.
Title: Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson’s disease.
Authors: Gasmi M, Herzog CD, Brandon EP, Cunningham JJ, Ramirez GA, Ketchum ET, Bartus RT.
Journal: Mol Ther. 2007 Jan;15(1):62-8.
PMID: 17164776 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers reported that when an AAV virus containing neurturin DNA was injected into the brain of rodents, levels of neurturin increased rapidly over 4 weeks and was stably produced for at least 1 year (the longest timepoint the investigators looked at). They also reported that the treatment delivered by the virus (called CERE-120) was bioactive, because it provided neuroprotection to dopamine neurons in a rodent model of Parkinson’s.
This treatment was also found to be neuroprotective in primate models of Parkinson’s (Click here and here to read more about this).
|
RECAP #3: Gene therapy approaches for neurturin were found to be neuroprotective in models of Parkinson’s. Researchers used Adeno-associated viruses (or AAVs) as the developed the treatment for humans because these viruses cause less response from the immune system.
|
CERE-120 was developed by a biotech company called Ceregene.
 And based on the encouraging preclinical research, the company initiated a Phase I clinical study in 6 people with Parkinson’s. The treatment was found to be safe and well tolerated (Click here to read the results of the study), so the company initiated a Phase II clinical trial (Click here to read the details of that study), and the results were published in 2012 (Click here to read those) with a longer-term follow up set of results being published in 2015:
And based on the encouraging preclinical research, the company initiated a Phase I clinical study in 6 people with Parkinson’s. The treatment was found to be safe and well tolerated (Click here to read the results of the study), so the company initiated a Phase II clinical trial (Click here to read the details of that study), and the results were published in 2012 (Click here to read those) with a longer-term follow up set of results being published in 2015:
 Title: Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial
Title: Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial
Authors: Warren Olanow C, Bartus RT, Baumann TL, Factor S, Boulis N, Stacy M, Turner DA, Marks W, Larson P, Starr PA, Jankovic J, Simpson R, Watts R, Guthrie B, Poston K, Henderson JM, Stern M, Baltuch G, Goetz CG, Herzog C, Kordower JH, Alterman R, Lozano AM, Lang AE.
Journal: Ann Neurol. 2015 Aug;78(2):248-57.
PMID: 26061140
In this study, between December 2006 and November 2008, 58 patients at 9 research sites across the USA were recruited to the trial. The participants were randomly assigned to either receive bi-lateral injections in the brain of an AAV-virus containing the DNA of neurturin, or a shame surgery. But after a 15- to 24-month study, the investigators found no difference between the participants.
In late 2013, the biotech firm Ceregene (and all of its intellectual property/patents) was acquired by the gene therapy company Sangamo (Click here to read the press release).
But to their great credit, the research scientists involved with the clinical trial, continued to follow up with many of the participants who took part in the trial. And as those individuals passed away (by natural causes), post-mortem analyses of their brains were undertaken.
This report provided some of the first results:
 Title: Post-mortem assessment of the short and long-term effects of the trophic factor neurturin in patients with α-synucleinopathies.
Title: Post-mortem assessment of the short and long-term effects of the trophic factor neurturin in patients with α-synucleinopathies.
Authors: Bartus RT, Kordower JH, Johnson EM Jr, Brown L, Kruegel BR, Chu Y, Baumann TL, Lang AE, Olanow CW, Herzog CD.
Journal: Neurobiol Dis. 2015 Jun;78:162-71. doi: 10.1016/j.nbd.2015.03.023. Epub 2015 Apr 2.
PMID: 25841760
In this study, the investigators conducted a post-mortem analysis on 4 participants involved in the Ceregene neurturin trial. They found that neurturin protein was being produced in 15-20% of the target region of interest (the putamen area of the brain) and the number of dopamine neuron branches increased with time since treatment, suggesting that the therapy may have been having an effect. But this response was less than what had been observed in the animal models.
The researchers concluded that their findings demonstrated mild but persistent production of gene therapy mediated neurturin for up to 4-years.
And that was the longest post-mortem timepoint assessed in the study… until this report was published this week:
 Title: Long-term post-mortem studies following neurturin gene therapy in patients with advanced Parkinson’s disease.
Title: Long-term post-mortem studies following neurturin gene therapy in patients with advanced Parkinson’s disease.
Authors: Chu Y, Bartus RT, Manfredsson FP, Olanow CW, Kordower JH.
Journal: Brain. 2020 Mar 1;143(3):960-975.
PMID: 32203581 (This report is OPEN ACCESS if you wouldl like to read it)
In this study, the researchers conducted postmortem analyses on sections of brain from participants in the study who have survived the longest to date (8 & 10 years post-surgery). The investigators found that neurturin was being produced in the putamen region of interest, but in only a limited area (covering ∼3-12% of the putamen).
But in the area where it was being produced, the researchers saw good evidence of dopamine branches still being present. In the image below, on the left-hand panel you can see a dark patch on the tissue where neurturin protein has been labelled with a special staining technique. In the right-hand panel, you can see the dark staining of dopamine branches in an area that overlaps with the neurturin stained region in the left hand panel.
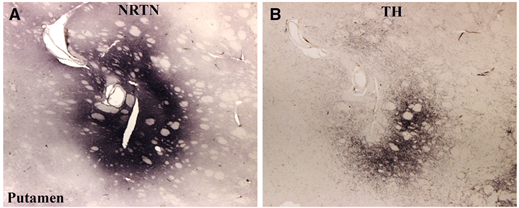 Source: Brain
Source: Brain
A closer examination of the dopamine neuron fibres in the neurturin producing region of the putamen (panel C and D in the image below) suggests a similar density of dopamine fibres to that seen in a normal putamen (panel A and B in the image below):
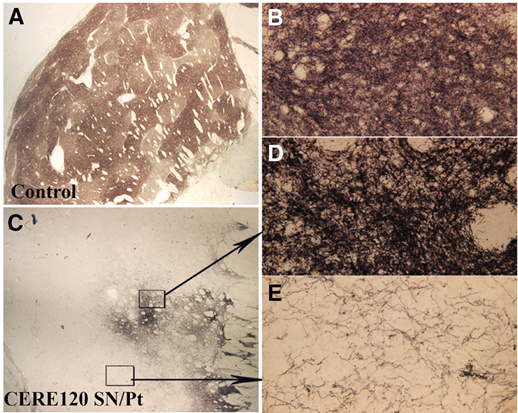 Source: Brain
Source: Brain
The result is very interesting when one compares the dopamine fibres in the neurturin producing region of the putamen with dopamine fibres in a region of the putamen that is not producing neurturin (Panel E in the image above).
But the researchers reported that these localised results were not associated with any clinical benefits. In addition, they found no difference between the tissue collected from the trial participants and control samples in terms of Parkinson’s-associated pathology, such as Lewy bodies.
The investigators concluded their report by stating that the results provide the longest term evidence of persistent gene therapy-based activity in the Parkinsonian brain, and offers some evidence for biological effect.
Is there any intention to repeat the study with more treatment covering a wider area of the region of interest?
I am not sure. The researchers do not discuss this in the report. And Sangamo – the biotech company that now owns the neurturin gene therapy approach – makes no mention of it on their pipeline page (but interesting to note that they do have an α-Synuclein-focused treatment under development with Biogen, called ST-502).
I am assuming that if there is interest in re-exploring the neurturin gene therapy treatment, it may be prudent to wait and see how things go with the GDNF gene therapy clinical trial currently being conducted by Brain Neurotherapy Bio.
 We have previously discuss this clinical trial and the early pilot results in a previous SoPD post (Click here to read that post).
We have previously discuss this clinical trial and the early pilot results in a previous SoPD post (Click here to read that post).
So what does it all mean?
I have taken part in clinical studies several times, but only ever acting as a control subject. I have never been in a position to be recruited as a treatment participant. I have enormous respect for anyone that steps forward to take part in such endeavours, but I equally admire the researchers who are prepared to be responsible for the safety and well being of these recruited individuals. It is a major undertaking for both parties, and it doesn’t finish when the trial completes its assessment period (as today’s post demonstrates).
Researchers have conducted post-mortem analyses on samples of brains from people with Parkinson’s who were recruited to test a gene therapy approach for the treatment of Parkinson’s. A new report explores samples from the longest period of survival to date (8 & 10 years post-surgery), and the results suggest not only that the gene therapy treatment is active, but also having some biological effect (though not enough for any clinical effect). These findings bode well for other gene therapy as an approach and provide useful information for studies that are currently being conducted.

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from cellculturedish.

Excellent post Simon. Is there anything to suggest that this might extend outside the dopaminergic system?
LikeLike
Hi Deepak,
It’s a great question. I don’t believe the researchers explored this in their report beyond looking at the long-term inflammatory reaction of CERE120 treatment and they did not see any differences. Might be worth dropping the authors an email and asking them directly.
Kind regards,
Simon
LikeLike
Simon, thank you for the very interesting post. I have two questions: 1. how does this clinical trial compare to NCT03562494?
https://clinicaltrials.gov/ct2/show/NCT03562494
2. Is there any research into application of micro doses of iboga that by some accounts promotes GDNF?
Thank you,
Felix
LikeLike
Hi Felix,
Thanks for your comment – glad you liked the post. The Voyager VY-AADC02 study is a dopamine replacement approach, while the Ceregene AAV-Neurturin trial was an attempt at disease modification. Both involve AAV virus-based gene therapy, but the Voyager approach is unlikely to slow or reverse PD, while the Ceregene was attempting to protect and restore the remaining dopamine neurons. We have previously discussed the Voyager effort ( https://scienceofparkinsons.com/2018/07/21/voyager-therapeutics-update/ ).
Regarding iboga – I get asked about ibogaine (an extract of iboga) a lot and here is my cut and paste answer: As far as I’m aware, no research has been done on ibogaine in the context of Parkinson’s. In addition, it is a compound that carries some serious warnings with it. Ibogaine’s use is restricted in the UK (Psychoactive Substances Act 2016) and completely banned in the US. Ibogaine can have serious side effects – hallucinations and dangerously lowering heart rate, plus it is pretty toxic for purkinje neurons (in the cerebellum). A clinical trial of ibogaine actually had a fatality at the higher doses (https://www.ncbi.nlm.nih.gov/pubmed/10506904).
Until more research is done in the context of PD (micro dose or otherwise), I can’t really comment.
I hope this helps.
Kind regards,
Simon
LikeLike