|
In the Parkinsonian brain, there is a severe reduction in a substance called dopamine. Reduced levels of this chemical are associated with the appearance of the motor features of Parkinson’s. Dopamine replacement therapies has been the front line therapy for the condition for the last 50 years. But long-term use of drugs like L-dopa are associated with the rise of motor complications, like dyskinesias. In the an effort to correct this, researchers in France have recently developed a method of continuously and directly delivering dopamine to the brain. They have now published the results of a study evaluating the safety and feasibility of this approach in a primate model of Parkinson’s. In today’s post, we will discuss what dopamine is, review the results of this new research, and explore what might happen next for this new potential treatment method.
|
 Prof David Devos. Source: Youtube
Prof David Devos. Source: Youtube
This is Dr David Devos.
He is Professor of medical pharmacology at University of Lille (France), world-renowned Parkinson’s researchers, a passionate advocate for the Parkinson’s community, and on top of all that he’s a really (and I mean REALLY) nice guy as well.
Recently, his research group (in collaboration with other scientists) published a report presenting a novel way of treating Parkinson’s, that he is now hoping to take to the clinic.
Here is the report:
 Title: Intraventricular dopamine infusion alleviates motor symptoms in a primate model of Parkinson’s disease.
Title: Intraventricular dopamine infusion alleviates motor symptoms in a primate model of Parkinson’s disease.
Authors: Moreau C, Rolland AS, Pioli E, Li Q, Odou P, Barthelemy C, Lannoy D, Demailly A, Carta N, Deramecourt V, Auger F, Kuchcinski G, Laloux C, Defebvre L, Bordet R, Duce J, Devedjian JC, Bezard E, Fisichella M, David D.
Journal: Neurobiol Dis. 2020 Mar 20:104846.
PMID: 32205254 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers wanted to explore how to directly deliver a chemical called dopamine to the brain.
What is dopamine?
Dopamine is a chemical is the brain that plays a role in many basic functions of the brain, such as motor co-ordination, reward, and memory.
It works as a signalling molecule – a way for brain cells to communicate with each other. Dopamine is released from neuron, and it binds to target neurons, initiating biological process within those cells.
In this manner, it is called a neurotransmitter.
 Dopamine being released by one cell and binding to another. Source: Truelibido
Dopamine being released by one cell and binding to another. Source: Truelibido
The dopamine neurons in the substantia nigra region of the brain generate the bulk of the dopamine in your brain, but they release most of it in different areas of the brain. The primary regions of that release are areas of the brain called the putamen and the caudate nucleus. The dopamine neurons of the substantia nigra have long projecting branches (or axons – the black curving lines in the image below) that extend a long way up into the brain to the putamen and caudate nucleus, so that dopamine can be released there.
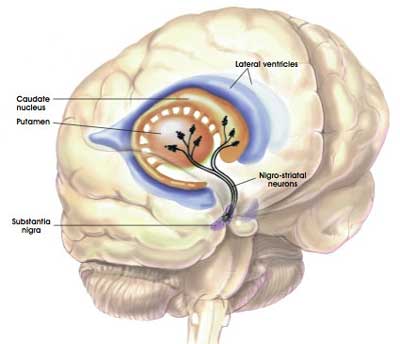
The projections of the substantia nigra dopamine neurons. Source: MyBrainNotes
In Parkinson’s, these ‘axon’ extensions that project to the putamen and caudate nucleus gradually disappear as the dopamine neurons of the substantia nigra are lost.
By the time a person is presenting the motor features characteristic of Parkinson’s, and being referred to a neurologist for diagnosis, they have already lost approximately 50% of the dopamine producing neurons in the substantia nigra.
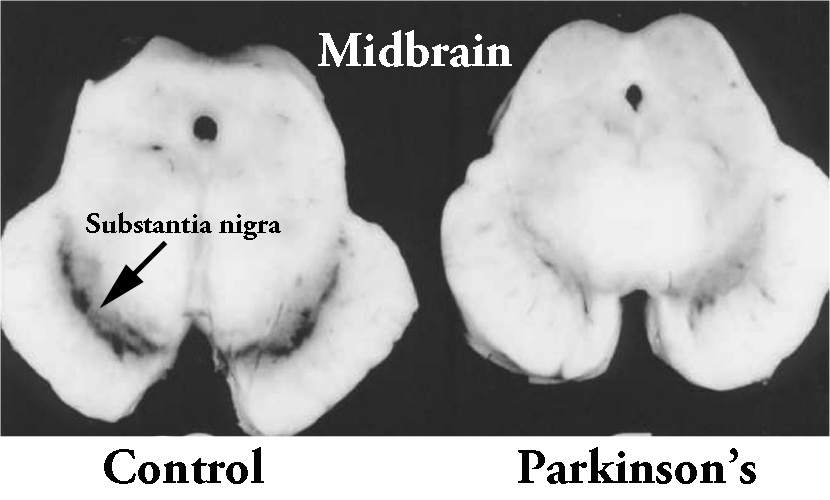 The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
This results in a reduction of dopamine being released in the putamen and caudate, which leaves the movement areas of the brain gradually becoming more inhibited. And this increase in inhibition shows itself clinically by the slowness of and problems initiating movement.
I see. So how do doctors currently treat Parkinson’s so that people can move around ok?
One of the primary treatments for Parkinson’s is levodopa (or L-dopa). It is an ingredient in the production of dopamine.
How does levodopa work?
When you take an L-dopa tablet, the pill will be broken down in your stomach and the L-dopa will enter your blood through the intestinal wall. Via your bloodstream, it arrives in the brain where it will be absorbed by cells. Inside the cells, another chemical (called DOPA decarboxylase) then changes it into dopamine. And that dopamine is released, and that helps to alleviate the motor features of Parkinson’s.

The production of dopamine, using L-dopa. Source: Watcut
Outside the brain, there is a lot of DOPA decarboxylase in other organs of the body, and if this is not blocked then the effect of levodopa is reduced in the brain, as less levodopa actually reaches the brain. To this end, people with Parkinson’s disease are also given Carbidopa which inhibits DOPA decarboxylase outside of the brain (Carbidopa does not cross the blood-brain-barrier, or the BBB in the image above which is a protective film/membrane covering the entire brain).
Ok, but why don’t we just use dopamine?
We can not use dopamine directly because:
- It can not cross the blood brain barrier (described above), and
- Dopamine is broken down very quickly in the body. There are lots of enzymes floating around that can digest dopamine so that it doesn’t do anything it’s not supposed to.
|
RECAP #1: Parkinson’s is characterised by the loss of a chemical called dopamine in the brain. By the time a person is diagnosed with Parkinson’s, they have typically lost half of their dopamine producing neurons. Neurologists treat Parkinson’s with dopamine replacement medication, which temporarily restores dopamine levels in the brain and allows sufferers to have a better quality of life.
|
You mentioned motor complications like dyskinesia. What are dyskinesias and how do they develop in Parkinson’s?
Dyskinesias (from Greek: dys – abnormal; and kinēsis – motion, movement) are a category of movement disorders that are characterised by involuntary muscle movements. And they are certainly not specific to Parkinson’s.
But in the case of Parkinson’s, dyskinesias have generally been believed to be associated with long-term use of levodopa (also known as Sinemet or Madopar – levodopa with carbidopa and levodopa with benserazide, respectively).

Sinemet is levodopa. Source: Drugs
NOTE: Long-term use of levodopa is not a certainty for developing dyskinesias, but there is an association. It will differ from person to person.
As for how they develop, there is a lot of debate over this topic, but there are some basic details that researchers generally tend to agree on.
Before being diagnosed and beginning a course of levodopa, the locomotion parts of the brain in a person with Parkinson’s gradually becomes more and more inhibited. This increasing level of inhibition results in the slowness and difficulty in initiating movement that characterises this condition.
A person with Parkinson’s may want to move, but they can’t – they are inhibited. In effect, they are akinetic (from Greek: a-, not, without; and kinēsis – motion).

Drawing of an akinetic individual with Parkinson’s, by Sir William Richard Gowers
Source: Wikipedia
Once inside the brain, levodopa is quickly converted into dopamine. It is changed into dopamine by an enzyme called DOPA decarboxylase, and this change rapidly increases the levels of dopamine in the brain, allowing the locomotion parts of the brain to function more normally.

The chemical conversion of L-DOPA to dopamine. Source: Nootrobox
In understanding this process, it is important to appreciate that when an levodopa tablet is consumed and levodopa enters the brain, there is a rapid increase in the levels of dopamine. This ‘spike’ in the supply of dopamine will last for the next few hours, before the dopamine is eventually used up.
As the effects of the levodopa tablet wear off, another tablet will be required. This use of multiple levodopa pills across the day gives rise to a wave-like shape to the dopamine levels in the brain over the course of the day (see the figure below). The first pill in the morning will quickly lift the levels of dopamine enough that the individual will no longer feel akinetic. This will allow them to be able to function with normal controlled movement for several hours before the levodopa begins to wear off. As the levodopa wears off, the dopamine levels in the brain drop back towards levels that will leave the person feeling akinetic and at this point another levodopa tablet is required.
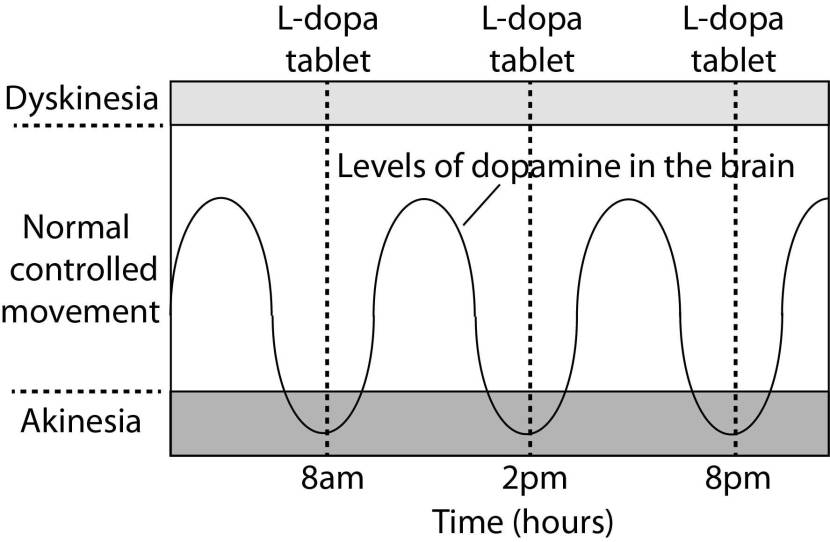
A hypothetical illustration of dopamine levels over a day
After several years of levodopa use, many people with Parkinson’s will experience a weaker response to each tablet. They will also find that they have more time during which they will be unable to move (exhibiting akinesia). This is simply the result of the slow progression of Parkinson’s – levodopa treats the motor features of the condition but only hides/masks the fact that the disease is still progressing.
To combat this shorter response time, the dose of levodopa is usually increased. This will result in increasing levels of dopamine in the brain (as illustrated by the higher wave form over time in the image below). Gradually it will take more levodopa medication-induced dopamine to lift the individual out of the akinetic state.

Again this illustration is hypothetical (situation differs between individuals)
This increasing of levodopa dosage, however, results in too much dopamine being present in the brain at times. And this situation is often associated with the gradual development of abnormal involuntary movements that appear when the levels of levodopa induced dopamine are the highest.
These are the involuntary muscle movements that we refer to as dyskinesias.
|
RECAP #2: Although it differs from person to person, long term use of levodopa therapy is associated with motor complications, such as dyskinesia. This may be partly due to the highs and lows of dopamine levels related to using pills forms of levodopa over the course of a day.
|
Would a more continuous delivery of dopamine help?
Research groups are exploring different approaches towards achieving a more continuous delivery of dopamine.
There is already a system of continuous delivery of levodopa, such as the Duodopa system, which injects a gel-based form of levodopa into the small intestine, where it can be absorbed into the blood stream.
 Duodopa. Source: Nature
Duodopa. Source: Nature
But recently, researchers have been exploring a more direct approach – that is to say, delivery of dopamine (rather than levodopa), and administering it directly into the brain, rather than the gut.
And this is where Prof Devos and his team come into the picture.
What did Prof Devos and his team report in their study?
Several years ago, Prof Devos and his colleagues published this report:
 Title: Continuous cerebroventricular administration of dopamine: A new treatment for severe dyskinesia in Parkinson’s disease?
Title: Continuous cerebroventricular administration of dopamine: A new treatment for severe dyskinesia in Parkinson’s disease?
Authors: Laloux C, Gouel F, Lachaud C, Timmerman K, Do Van B, Jonneaux A, Petrault M, Garcon G, Rouaix N, Moreau C, Bordet R, Duce JA, Devedjian JC, Devos D.
Journal: Neurobiol Dis. 2017 Jul;103:24-31.
PMID: 28363801 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers demonstrated that continuous delivery of a stable form of dopamine (called anaerobically prepared dopamine or “A-dopamine”) could restore function in neurotoxic models of Parkinson’s (MPTP mice and 6-OHDA rats) when delivered directly into the brain.
They also reported that the treatment did not cause dyskinesias, which were observed with orally administered levodopa treatment. The investigators concluded that this approach could be very useful in treating people with Parkinson’s.
But before beginning clinical trials in humans, the health system regulators require non-human primate evaluation to assess safety. And the new report published this week provides the results of that study.
Here is the report:
 Title: Intraventricular dopamine infusion alleviates motor symptoms in a primate model of Parkinson’s disease.
Title: Intraventricular dopamine infusion alleviates motor symptoms in a primate model of Parkinson’s disease.
Authors: Moreau C, Rolland AS, Pioli E, Li Q, Odou P, Barthelemy C, Lannoy D, Demailly A, Carta N, Deramecourt V, Auger F, Kuchcinski G, Laloux C, Defebvre L, Bordet R, Duce J, Devedjian JC, Bezard E, Fisichella M, David D.
Journal: Neurobiol Dis. 2020 Mar 20:104846.
PMID: 32205254 (This report is OPEN ACCESS if you would like to read it)
In this study, the researchers used 8 primates that had Parkinson’s-like features induced using the neurotoxin MPTP, and they implanted a catheter (a tube for delivering solutions) into the ventricular system of the brain.
What are “ventricular system of the brain”?
Inside your brain, there are some empty spaces which are filled with fluid. These spaces are called the ‘ventricles‘ or the ventricular system of the brain.
In the human brain there are 4 basic divisions of the ventricles as you can see in the image below (the ventricles are the yellow space):

The ventricles in the human brain (yellow coloured regions). Source: PhysRev
The ventricles are filled up with a solution called cerebrospinal fluid. Cerebrospinal fluid is a clear, colorless fluid that is very similar to the liquid portion of blood (or plasma – if you remove the cells from blood, it’s called plasma), except that cerebrospinal fluid is nearly devoid of protein.
Cerebrospinal fluid protects the brain against injury when suddenly jolted or hit. It also allows for the removal of waste from the brain, washing it away. But ciritically, cerebrospinal fluid allows for the distribution of molecules between cells of the brain.
And this is where the researchers were hoping that by putting the stable form of dopamine into the ventricular system of the brain, it would be able to get to all the places it is supposed to go.
And did it?
Yes, it did.
Over the course of 60 days of a continuous delivery of “A-dopamine”, the researcher observed an improvement in the motor and cognitive symptoms of the treated monkeys (with a therapeutic window of 30 to 70 mg/day). And the investigators observed no tachyphylaxia (which is a diminishing response to continuous delivery of a drug, basically rendering it less effective) over the study.
Importantly, no dyskinesia was observed during the continuous delivery of “A-dopamine” (even at very high doses).
Postmortem analysis of the animals found no abnormalities in the brain or any of the other organs (heart, lungs, liver, kidney).
In conclusion, the researchers suggested that these results support the feasibility of this treatment route and demonstrated the efficacy (without dyskinesia) within a safe therapeutic window.
|
RECAP #3: Continuous delivery of dopamine into the brain resulted in behavioural improvements in models of Parkinson’s, without the occurence of motor complications like dyskinesias. The researchers concluded that the results support the clinical feasibility of this treatment route.
|
Has anyone ever tried direct delivery of dopamine into the brain before?
Yes they have, but given the unstable nature of dopamine, the results
 Title: Treatment of severe Parkinson’s disease by intraventricular injection of dopamine.
Title: Treatment of severe Parkinson’s disease by intraventricular injection of dopamine.
Authors: Venna N, Sabin TD, Ordia JI, Mark VH.
Journal: Appl Neurophysiol. 1984;47(1-2):62-4.
PMID: 6508279
In 1981, researchers placed a catheter into the brain of a 65-year old man who had lived with Parkinson’s for 17 years. The gentleman had become bedridden and was displaying the first symptoms of dementia. levodopa treatment had been exhausted and even low doses of the drug resulted in hallucinations (believed to be due to targets not related to dopamine).
While the procedure proved to be safe and minor movement improvements were reported, high doses of dopamine were not tested because the gentleman became confused with increased doses and the researchers were worried about the potential for dopamine oxidation inducing toxicity (this is where normal dopamine which is naturally unstable, breaks down and becomes toxic).
A second example of direct delivery of dopamine was reported a few years later:
 Title: Intraventricular infusion of Dopamine in Parkinson’s disease.
Title: Intraventricular infusion of Dopamine in Parkinson’s disease.
Authors: Horne MK, Butler EG, Gilligan BS, Wodak J, Stark RJ, Brazenor GA.
Journal: Ann Neurol. 1989 Dec;26(6):792-4.
PMID: 2604387
In this study, a patient with long-term Parkinson’s and “troublesome fluctuations in motor function” was treated with a direct ventricular infusion of dopamine in an effort to reduce those fluctuations. All oral treatment was withdrawn and the individual “became profoundly rigid and immobile”. But this improved as the rate of infusion of dopamine increased. Ultimately, he was “able to walk unaided for most of the day and achieved a clinical response similar to that produced by orally administered levodopa. His speech was clear and lucid and he had no dyskinesia“. As the doses increased, the individual experienced depression, confusion, and agitation, but these resided after the dose was lowered again.
But again, the unstable nature of dopamine was a worry for the researchers.
The new version of ‘A-dopamine’ (used in the new studies from France) has been shown to be more stable than normal dopamine.
Sounds interesting. So what is going to happen next with this research?
A biotech company called InBrain Pharma has been set up to further develop this research.
The company has been foundered up by Dr Matthieu Fisichella (CEO), Dr Caroline Moreau, and Prof Devos (in the image below, respectively).
 Source: lavoixdunord
Source: lavoixdunord
No word yet on clinical trials, but we will be following their progress closely here at the SoPD.
So what does it all mean?
Quite often here on the SoPD, I get excited by the biology underlying novel potentially disease modifying methods of treatment. Complicated molecular pathways that may slow down the progression of the condition, but could also carry unforeseen side-effects as well. Years away from being clinically available, and further refinement might be required proper utility, these discoveries are what really interest me as a researcher scientist.
It is also encouraging, however, to see the research community providing yet further improvements of ‘old’ treatment methods (such as dopamine replacement) – not simply minor adjustments for the purpose of maintaining profit streams, but real innovation allowing for an improved version of the old treatment approaches, that could have serious impact on providing a better quality of care for the community
Positive stuff for trying times like these.
Be safe.

All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from neuro-central


4 thoughts on “Direct dopamine delivery”