|
San Diego-based biotech firm Aspen Neuroscience recently announced that it has raised US$70 million in Series A funding to help its efforts to develop the first autologous neuron replacement therapy for treating Parkinson’s. Cell replacement therapy represents a treatment approach that carries a lot of hope for the Parkinson’s community – providing new cells for the ones that have degenerated in the condition, and taking up lost function. In today’s post, we will explore what “autologous neuron replacement therapy” means, look at what Aspen Neuroscience is doing, and discuss what will happen next.
|
In the SoPD ‘Road Ahead’ post at the start of this year (in which we discussed what is planned for Parkinson’s research in 2020 – click here to read that post), I briefly mentioned a biotech firm called Aspen Neuroscience.
It was one of the companies that I was going to be watching this year for signs of progress and development. I had no expectations, but was interested in what they are working on because it is in a rather exciting area of Parkinson’s research.
What does Aspen Neuroscience do?
The company works with stem cells.
It was co-founded by stem cell scientist Prof Jeanne Loring:
 Prof Jeanne Loring. Source: SDT
Prof Jeanne Loring. Source: SDT
She is a leading expert in the field of stem cell biology. Here is a video of Prof Loring talking about the potential of induced pluripotent stem cells:
What are induced pluripotent stem cells?
Pluripotent stem cells are cells that can be grown (in cell culture) to become any type of cell in the body – heart, brain, bone, blood, etc. ‘Pluripotent’ meaning capable of any fate. These cells start off in a naive state, but using specific protocols (or recipes) of certain proteins, these cells can be encouraged to differentiate into any kind of cell we may require.
 The menu. Source: Wikipedia
The menu. Source: Wikipedia
But Prof Loring was talking about the potential of “induced” pluripotent stem cells – what is meant by “induced”?
This is Prof Shinya Yamanaka.

Prof Shinya Yamanaka. Source: Glastone Institute
He’s a rockstar in the biomedical research community.
Prof Yamanaka is the director of Center for induced Pluripotent Stem Cell Research and Application (CiRA); and a professor at the Institute for Frontier Medical Sciences at Kyoto University.
But more importantly, in 2006 he published a research report that would quite literally ‘change everything’.
This is the report:

Title: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors.
Authors: Takahashi K, Yamanaka S.
Journal: Cell. 2006 Aug 25;126(4):663-76.
PMID: 16904174 (This article is OPEN ACCESS if you would like to read it)
In this study, Prof Yamanaka‘s team demonstrated a method by which someone could take a simple skin cell (called a fibroblast), grow it in cell culture for a while, and then re-program it so that it would transform back into a stem cell – a cell that is capable of becoming any kind of cell in the body.
So rather than just being a skin cell that could divide into other skins cells, the transformed ‘stem cell’ could now become any kind of cell.
And because they had persuaded the skin cells to change back to this new state, the researchers decided to call the transformed cells were called induced pluripotent stem (or IPS) cells.
Prof Yamanaka‘s team started their investigation with the basic hypothesis that proteins which are important to the maintenance of embryonic stem cells (the cells that give rise to all of the cells in your body) might also be able to cause an embryonic state in mature adult cells. They selected twenty-four proteins that had been previously identified as important in embryonic stem cells to test this idea. They used re-engineered viruses to deliver these proteins to mouse skin cells. The viruses were emptied of all their disease causing properties, and could thus function as very efficient biological delivery systems.
The skin cells were genetically engineered in such a fashion so that only cells in which reactivation of the embryonic stem cells-associated protein, Fbx15, would survive the testing process. If Fbx15 was not turned on in the cells, over time they would die. When the researchers infected the cells with all twenty-four embryonic stem cells genes, remarkably some of the cells actually survived and began to divide like stem cells.
In order to identify which proteins were necessary for the reprogramming, the researchers began removing one protein at a time from the pool of twenty-four. Through this process, they were able to narrow down the most effective proteins to just four: Oct4, Sox2, cMyc, and Klf4, which became known as the “Yamanaka factors”.
Wow! Interesting. But why is this important?
Understand that this was more than an amazing feat of molecular biology. It suddenly made the hypothetical idea of ‘personalised medicine’ very possible – take skin cells from anyone with a particular medical condition, turn them into whatever cell type you like, and then either test drugs on those cells or transplant them back into their body (replacing the cells that have been lost due to the medical condition).
 Personalised medicine with IPS cells. Source: Bodyhacks
Personalised medicine with IPS cells. Source: Bodyhacks
IPS cells are now being used all over the world, for all kinds of biomedical research. And many research groups are rushing to bring IPS cell-based therapies to the clinic in the hope of providing the long sort-after dream of personalised medicine.
And in acknowledgement of this incredible bit of research, in 2012 Prof Yamanaka and Prof John Gurdon (University of Cambridge) were awarded the Nobel prize for Physiology and Medicine for the discovery that mature cells can be converted back to stem cells.

Prof Yamanaka and Prof Gurdon. Source: UCSF
Prof Gurdon achieved a similar feat to Yamanaka’s work in 1962 when he removed the nucleus of a fertilised frog egg cell and replaced it with the nucleus of a cell taken from a tadpole’s intestine. That is to say, the nucleus of a ‘mature’ cell was placed into a frog egg cell, and guess what happened? The modified egg cell then grew into an adult frog! It started dividing and all of the off-spring cells began differentiating, giving rise to a fully functioning organism.
This fascinating research proved that the mature cell still contained the genetic information needed to form all types of cells.
EDITOR’S NOTE: I do not want to be accused of taking anything away from Prof Gurdon’s contribution to this field (which was great!) by not mentioning his efforts here. For the sake of saving time and space, we are focusing on Prof Yamanaka’s research as it is more directly related to today’s post.
Now, as I suggested above, the amazing discovery of IPS cells has opened new doors for biological research and provided us with incredible opportunities for therapeutic treatments.

Making IPS cells. Source: learn.genetics
For example, we can now take skins cells from a person with Parkinson’s and turn those cells into dopamine neurons which can then be tested with various drugs to see which treatment is most effective for that particular person (personalised medicine in it’s purest form).

Some of the option available to Parkinson’s disease. Source: Nature
Some of those dopamine neurons could also potentially be transplanted back into the person – into the brain to replace the lost dopamine neurons. This process would hopefully reduce the need for medication that suppresses the immune system, because the body would recognise the cells being transplanted as ‘self’ (or derived from the person being transplanted).
The process of taking cells from a person and then transplanting them back into that same individual is called autologous cell transplantation.
And this is what Aspen Neuroscience is planning to do: autologous cell transplantation of dopamine neurons made from a person’s own IPS cells.
|
RECAP #1: Induced pluripotent stem (or IPS) cells allow researchers to take a skin cell from any individual, grow them in cell cuture, and then re-program them back into a naive stem cell – which is capable of becoming any type of stem cell. These IPS cells can then be encouraged to become any kind of mature cell type, which can be used for personalised medicine – for example, testing drugs that would be specific for each person.
|
Why would we transplant dopamine cells into the brain?
By the time a person is presenting the motor features characteristic of Parkinson’s, and being referred to a neurologist for diagnosis, they have already lost approximately 50% of the dopamine producing neurons in an area of the brain called the midbrain.
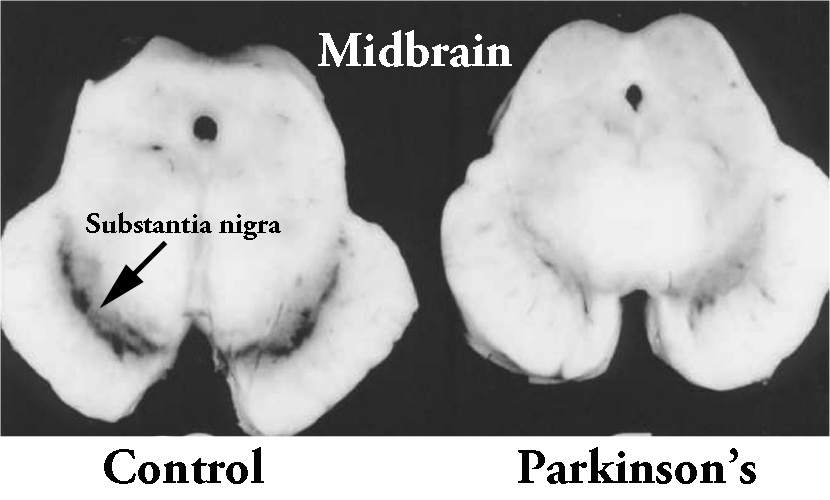 The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
The dark pigmented dopamine neurons in the substantia nigra are reduced in the Parkinsonian brain (right). Source:Memorangapp
These cells are critical for normal motor function – without them, movement becomes very inhibited.
And until we have developed methods that can identify Parkinson’s long before these cells are lost and the motor features appear, some form of cell replacement therapy is required to introduce new cells to take up the lost function.
Cell transplantation represents the most straight forward (but still experimental) method of cell replacement therapy.
How does cell transplantation work?
This ‘old fashioned’ approach to cell transplantation has involved dissecting out the region of the developing dopamine neurons from a donor embryo, breaking up the tissue into small pieces that could be passed through a tiny syringe, and then injecting those cells into the brain of a person with Parkinson’s.

The old cell transplantation process for Parkinson’s. Source: The Lancet
Critically, the people receiving this sort of transplant have required ‘immunosuppression treatment’ for a long period of time after the surgery. This additional treatment involves taking drugs that suppress the immune system’s ability to defend the body from foreign agents. This step is necessary, however, in order to stop the body’s immune system from attacking the transplanted cells (which would not be considered ‘self’ by the immune system), allowing those cells to have time to mature, integrate into the brain and produce dopamine.
The transplanted cells are injected into an area of the brain called the putamen.
What is the putamen?
The bulk of the dopamine neurons in the brain reside in an area called the substantia nigra, near the base of the brain, but they project their branches (or axons) to the several other areas, including the putamen, and this is where they release most of their actual dopamine (the chemical which helps us to move properly).
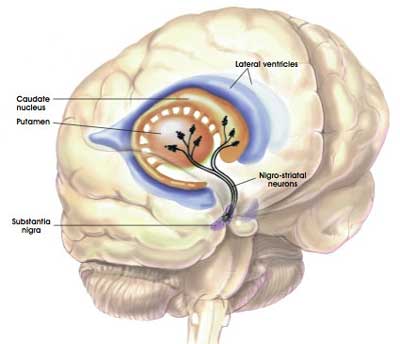 The projections of the substantia nigra dopamine neurons & location of the putamen. Source: MyBrainNotes
The projections of the substantia nigra dopamine neurons & location of the putamen. Source: MyBrainNotes
In people with Parkinson’s, the amount of dopamine being released in the putamen decreases over time. The image below demonstrates the loss of dopamine (the dark staining) over time as a result of Parkinson’s (PLEASE NOTE that the time scale presented here varies from person to person):
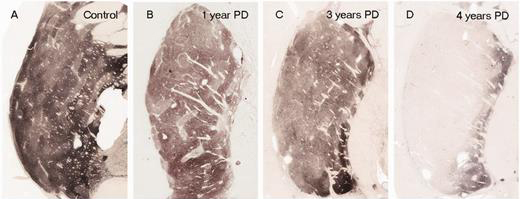
The loss of dopamine in the putamen as Parkinson’s progresses. Source: Brain
In cell transplant procedures for Parkinson’s, multiple injections of cells are usually made in the putamen, allowing for deposits in different areas of the structure. These multiple sites allow for the transplanted cells to produce dopamine across the entire extent of the putamen. And ideally, the cells should remain localised to the putamen, so that they are not producing dopamine in areas of the brain where it is not desired (possibly leading to side effects).

Targeting transplants into the putamen. Source: Intechopen
Postmortem analysis of the brains of individuals who have previously received transplants of dopamine neurons (and then subsequently died from natural causes) has revealed that the transplanted cells can survive the surgical procedure and integrate into the host brain. In the image below, you can see rich brown areas of the putamen in panel A. These brown areas are the dopamine producing cells. A magnified image of individual dopamine producing neurons (their circular bodies and their branches are stained in brown) can be seen in panel B:

Transplanted dopamine neurons. Source: Sciencedirect
The transplanted cells take several years to develop into mature neurons after the transplantation surgery. This means that the actually benefits of the transplantation technique will not be apparent for some time (2-3 years on average). Once mature, however, it has also been demonstrated (using brain imaging techniques) that these transplanted cells can produce dopamine.
As you can see in the images below, there is less dopamine being processed (indicated in red) in the putamen of the Parkinsonian brain on the left than the brain on the right (several years after bilateral – both sides of the brain – transplants):

Brain imaging of dopamine processing before and after a successful transplantation. Source: NIH
In some cases the individual being transplanted has been able to reduce the amount of Levodopa treatment that they take over time. As the transplanted cells start to produce enough dopamine in the right area of the brain, it allows the individual to function better and require less medication.
So this is what Aspen Neuroscience is planning to do: Use a person’s IPS cells to grow dopamine neurons that can then be transplanted into the brain.
Here is a video from last year of Prof Loring explaining it all is a bit more detail:
This sounds great! Like a cure for Parkinson’s?
Yeah, not quite.
Cell transplantion should not be considered as a “cure”. It is not a disease modifying approach, as it will not slow or stop the progression of the underlying biology of Parkinson’s.
Rather, cell transplantation should be viewed as a ‘restorative’ method, that provides dopamine replacement. And ideally as the transplanted neurons mature in the brain, they will produce increasing amounts of the chemical dopamine which will allow for reductions in dopamine-based medications over time.
|
RECAP #2: Cell transplantation is an experimental approach that represents a form of restorative therapy. A method of replacing some of the cells that have been lost in the course of Parkinson’s. Aspen Neuroscience proposes to take skin cells from people with Parkinson’s and turn them into IPS cells, which can then be differentiated into dopamine neurons that can be transplanted into their brains (reducing the need for immune system repressing medication).
|
How did Aspen Neuroscience start?
Good question – because there is an interesting story behind the company.
Prof Loring co-founded Apsen Neuroscience in late 2018 with Dr Andres Bratt-Leal, a former post-doctoral researcher in Loring’s lab at the Scripps Research Institute.
 Prof Loring with Dr Andres Bratt-Leal. Source: SDT
Prof Loring with Dr Andres Bratt-Leal. Source: SDT
To get the biotech off the ground, they were initially supported by an organisation called Summit for Stem Cell.
 Source: Summit for Stem Cell
Source: Summit for Stem Cell
And this is where the story gets inspiring.
Summit for Stem Cells is a non-profit was set up by a group of people with Parkinson’s, initially led by a nurse practitioner at the Scripps Clinic named Sherrie Gould.
 Source: Sherriegould
Source: Sherriegould
They have raised large pools of funding to help the stem cell research, and they did this by taking on climbing goals (hence the “Summit” part of the name).
So far they have done:
2016 – Macchu Picchu: 29 trekkers, nine with Parkinson’s. All succeeded over the 15,300 foot Salkanty pass and made it to Machu Picchu.
2018 – Camino de Santiago, Spain. 29 trekkers, 12 with Parkinson’s.
So inspiring were these efforts that the group grew into a large organisation with supporters all over the world.
Here is a video of Sherrie Gould discussing what is required:
I like her spirit (attitude really is everything).
Aspen Neuroscience sought seed funding in 2019 (led by Domain Associates and Axon Ventures, with additional participation from Alexandria Venture Investments, Arch Venture Partners, OrbiMed and Section 32 – click here to read the press release).
And then this week the company received further funding in the form of series A funding to “support the completion of all remaining Investigational New Drug (IND)-enabling studies and FDA submission of the IND relating to Aspen’s lead product” (Click here to read the press release).
This round of funding was led by OrbiMed (who are supporting many of the Silverstein Foundation projects) with participation from ARCH Venture Partners, Frazier Healthcare Partners, Domain Associates, Section 32, and Sam Altman.
Aspen Neuroscience is being led by CEO Prof Howard Federoff:
 Prof Howard Federoff. Source: OC
Prof Howard Federoff. Source: OC
“Our mission at Aspen Neuroscience is to develop a restorative, disease-modifying autologous neuron therapy for people suffering from Parkinson’s disease. We are dedicated to using a person’s own cells for replacement therapy and bringing best-in-class treatments to people suffering from Parkinson disease as rapidly as possible.”
– Dr. Howard Federoff (Source)
Dr Federoff is a Professor of Neurology at University of California, Irvine, College of Medicine where he was previously the Dean. Importantly, he has a great deal of experience with clinical trials and biotechs focused on Parkinson’s. For example, he was a co-founder of two biotech companies that are focused on taking GDNF through to the clinic for Parkinson’s (MedGenesis Therapeutix and Brain Neurotherapy Bio – click here and here to read previous SoPD posts involving these companies).
In addition, he is a really nice guy.
NOTE: The author of this blog is acquainted with Prof Federoff as he is also a member of the international Linked Clinical Trials committee that The Cure Parkinson’s Trust co-ordinates (with our friends at the Van Andel Institute – click here to read a previous SoPD post discussing the work of this committee).
|
RECAP #3: Aspen Neuroscience was initially supported by a non-profit, organised by individuals in the Parkinson’s community. Angel and venture capitalists have now stepped forward to support the company as they prepare for initiating clinical trials of their lead product.
|
Ok, so what happens next?
As mentioned above, the company is completing the Investigational New Drug (IND)-enabling studies and FDA submission of their lead product, which is called ANPD001.
ANPD001 is an autologous cell transplantation therapy which is being developed for sporadic Parkinson’s (that is, cases of Parkinson’s without any obvious genetic associations).
Source: AspenBut the company is also working on a second product, conveniently called ANPD002, which is a combination of gene correction technology and autologous cell transplantation therapy. This product is being developed for the treatment of genetic forms of Parkinson’s (particularly GBA-associated Parkinson’s).
The idea here is that a person with Parkinson’s who also carries a genetic variant (like GBA) will provide a sample of their skin cells, those cells will be grown and genetically corrected, before they are then grown into dopamine neurons for transplantation. This way the cells being transplanted will be healthier and stronger.
The company states on their website that they will be seeking FDA Orphan Designation for this product, which should help with the clinical trial process.
 Source: Aspen
Source: Aspen
With the recent series A funding, Aspen Neuroscience will also be seeking to recruit and screen a trial-ready cohort of people with Parkinson’s for a safety and tolerability Phase 1 clinical trial. This will hopefully provide the company with useful data, laying the ground work for a larger Phase 2 multi-center randomised controlled trial.
Very interesting. Is Aspen Neuroscience the only biotech doing this?
COVID-19 withstanding, 2020 is an going to be a very active year for cell transplantation projects.
In addition to Aspen Neuroscience, there is also a biotech company called BlueRock Therapeutics (which was recently bought by the Pharmaceutical company Bayer – click here to read a previous SoPD post about this).
In addition, to this there is a lot of noise in the jungle from other companies seeking to enter the cell transplantation space (such as Fujifilm/Cellular Dynamics or Novo Nordisk).
It will be interesting to see if they step up to the plate and start a clinical trial program focused on cell transplantation for Parkinson’s now that Aspen Neuroscience is moving forward.
The major difference between what Aspen are planning and these other contenders have suggested that they will do, is that Aspen is focused on autologous cell transplantation therapy. Other parties (such as the Kyoto study – click here to read more about this) are starting with allogeneic IPS cell approaches. This means that they will use a common pool of IPS cells to conduct their initial trials.
 Source: Aspen
Source: Aspen
The advantage to the allogeneic approach is that one can have a ready supply of cells always available. The allogenic method will require a longer period of preparation time as an individuals cells are grown, converted, and differentiated. This will require weeks to months to grow enough cells for transplantation.
The disadvantage of the allogeneic approach is that immuno suppression medication will be required (because the cells are not ‘self’, the immune system will attack them and extended use of immuno suppressants will be required to give the cells an opportunity to grow and survive). There is also the opportunity for spontaneous genetic mutations to appear in allogeneic pools of continuously expanded cells. Continuous monitoring will be required as the cells are being grown in culture.
So what does it all mean?
The Parkinson’s community holds a lot of hope in stem cell-based therapies as a means of helping to restore some of the lost function. Recently, research in this field has arrived at a point where the scientists have finally developed protocols that they are confident with for the production of the right types of cells. And as a result there are a number of parties moving these research efforts towards the clinic.
Aspen Neuroscience is taking a very ambitious approach by proposing to take skin cells from people with Parkinson’s, re-program them to become IPS cells, and differentiate them before transplanting them into the brains of those individuals. Not an easy task, and this will be a long game (those transplanted cells will take 2-3 years to mature and integrate into the brain).
But Aspens activities represents a significant development in the area of restorative therapy for the Parkinson’s community and it will certainly command a lot of attention going forward.
Fingers crossed all goes well for Aspen with their aspirations.
All of the material on this website is licensed under a
Creative Commons Attribution 4.0 International License
You can do whatever you like with it!
The banner for today’s post was sourced from Aspen Neuroscience.





Exciting times ahead, Simon. Either this or AADC gene therapy (with external titration of ldooa of course)or both. We’re going to see a drastic uptick in quality of life metrics with the arrival of stem cell / gene therapy approaches for the motor symptoms. Let’s not forget that transeuro results are due next year. These approaches will add decades of good QoL, In which time I’m sure we’ll find a disease arresting mechanism.
Any idea when Aspen plan on dosing their first patients? Excellent post as always Simon. Stay safe. And thanks for everything you do
LikeLike
Hi Deepak,
Thanks for your comment and I hope that you are right about improving QoL, but as usual here at the SoPD, we have no expectations. What I will say is that patient selection will be crucial in the transplantation studies (Aspen, Bluerock/Bayer, etc). The research community is worried about dyskinesias as this has been an issue with previous transplantation studies. It will be interesting to see what the Aspen study protocol says about this when they announce the first study. I have no information regarding the details of their phase 1 trial yet, but they will be focused on safety first. If the FDA gives the green light on their IND, we will hopefully learn more soon.
Kind regards,
Simon
LikeLike
True, Simon. However I’m basing my confidence on some of the post transplant studies done on dyskinesia where serotonin agonists like buspirone were able to adequately get rid of dyskinesias, leading to the hypothesis that the dyskinesias were perhaps being caused by the presence of serotonin neurons in the fetal grafts. So I’m not absolutely sure, but I’m cautiously confident. The transuro team has been careful to dissect out the serotonin neurons to the greatest extent possible, and so we’ll know next year when the results are out for that study if that hypothesis holds any validity. And if that hypothesis is correct, then stem cell derived neuron transplants automatically address the issue. Only time will tell.
I listened to a magnificent lecture by Lorenz Studer where he says that his team in New York will likely exclude patients who already suffer from ldopa induced dyskinesia.
LikeLike
There always some hope with Aspen protocol to transplant autologus dopamin neuron to putamen. But if the misfolded alpha synuclein still active as prion as we know even as current situation they degrade the remaining dopamin neuron in the brain of PD patient. This misfolded alpha synuclein should be consider how to tackle it before the transplantation
LikeLike
Baby steps. You’re right in that the transplant of neurons will eventually acquire lewy pathology. However this has been shown to take decades to occur, and a majority of the patients will probably die of other reasons, considering that most patients are over 60 years of age at initial diagnosis. So this is effectively a cure for the motor symptoms.
However, I agree with you that this will need some addressing. There are potential gene therapy approaches with plans to encode genes for gdnf, and perhaps even monoclonal antibodies to deal synuclein clumps INSIDE the neuron. Voyager Therapeutics and AbbVie are trying to come up with a gene therapy for doing just that.
LikeLiked by 1 person
Incidentally the partnered with a company called Scaled Biolabs (https://scaledbiolabs.com/#overview) . I could be wrong but my understanding of this partnership is that scaled have a platform that, combined with Jean Loring’s extensive background in genomics, will lead to inexpensive and safe autologous dopaminergic neuron manufacture. Many experts including Roger Barker and Lorenz Studer are of the opinion that autologous therapy will be only for the super rich. We’re all behind Dr. Loring to bring this to the clinic and improve patient QoL
LikeLike
It is well known that stem cells cured Parkinson’s disease in Sweden in the 1980s (at least some people). They never needed to take parkinson medicins again. But I wonder how long it will take before these new treatments are available. I am shocked to see how fast research can suddenly go with Covid-19. A lot of steps are skipped to test treatments. Why isn’t the same done for people with neurological conditions ? It is truely ridiculous to realize that in the 1980s some people were cured from PD using stem cells and that now 40 years later we still are not able to cure PD patients with stem cells.
LikeLike
That those fetal cells cured Parkinson’s is a bold claim, for a cure means that the underlying disease process completely gets eliminated. It certainly reset the hourglass in the dopaminergic system, but then in several situations the grafts themselves acquired lewy pathology. Also Parkinson’s is more than just the dopamine system. Although I must admit that the dopamibe symptoms are the most prevalent and by far the most debilitating from a quality of life standpoint. What these therapies, including gene therapies, can do are to drastically better the quality of life for two or three decades until Parkinson’s invariably kicks in and causes autonomic dysfunction, sleep issues, falls etc. Two decades is a long time from a clinical trials perspective. Long enough for a new therapeutic to come in that will alter the course of the disease. Stay strong people
LikeLiked by 1 person
Theoretically they weren’t cured because the disease process was still there. But from a practical point of view they were cured. They never had to take anti PD meds again. PD is a slow process. If you can give patients back the 80 % lost dopamine neurons, then they will never have problems again.
LikeLiked by 1 person
Hi Diego,
Thanks for your comment.
I agree with you that it is curious that science is able to bend the rules/break down barriers when there is an urgent need, but how a brain tumor, ALS or Parkinson’s isn’t ‘urgent’ baffles me (I have a SoPD rant in waiting discussing this dichotomy).
I disagree with you, however, on your transplantation statements. And I am very familiar with the Swedish transplantation clinical trials because I did my PhD in the research group that conducted those studies. Very few of the original participants were able to come off medication completely. An good example in the literature was a case study published in 2016 – the longest survival period analysis to date as far as I’m aware (https://www.pnas.org/content/early/2016/04/26/1605245113.full?sid=d3ca3a44-754e-406b-a788-14f30bc34a32). This report provides a lot of clinical details post-transplantation and the procedure was certainly not a ‘cure’. Nor did the participant ‘never need to take Parkinson’s medicine’ ever again. In this individual (which was one of the more successful cases from the Lund studies), L-dopa treatment was withdrawn at 32 months post surgery, but was re-introduced at 74 months. From there, “motor function remained unchanged until 12 years posttransplantation, when a low-dose dopamine agonist was added for 2 years and the l-dopa dose was increased to the preoperative level because of worsening hypokinesia”. The individual passed away due to cardiac issues 24 years after transplantation.
Many in the research community (myself included) consider the current versions of cell transplantation as a symptomatic therapy because it is simply designed as a dopamine replacement treatment. It is certainly not disease modifying. But if it can reset the hour glass (as Deepak eloquently put it), then yes that will hopefully help slow down the progression of the motor features as the transplanted cells slowly mature and take over from the the lost dopamine neurons. What is important for the community to understand is that this treatment approach should not be seen in isolation (we need 3 components for a cure: a disease halting therapy, a neuroprotective approach, and cell replacement method). Future therapy for PD will involve a multi-modal approaches, but this is still some ways off.
Kind regards,
Simon
LikeLiked by 2 people
Simon, thanks for that link. Why in your opinion did that graft stop working after 12 years and under 5% of the grafted neurons acquiring pathology? Also It seems a little counterintuitive that these neurons stopped working, but then postmortem there was rich innervation.
Finally, how did this patient do from a non-motor standpoint?
LikeLike
Thanks for the reply. Looking forward to your rant 🙂
I know that stem cells don’t stop the underlying disease process. But it would make life way much easier for PD patients. Now when you take a levedopa pill you have to wait until it works, you need to be careful what you eat or your pill doesn’t work well, and after a while the pill stops working. There is also the problem of diskenesia when at advanced stage. With proper working stem cells all these problems can be eliminated. Your level of dopamine is constant and you get it the whole day, so no more off periods, no diskenesia.
Many people also underestimate the problem of taking a levedopa pill. My father has PD and one of his biggest issues is what to eat and when to eat. He can’t eat normal anymore. He lost a lot of weight because he has problems eating proteins because it messes with the levedopa pill. For example, he always has to eat a small portion of fish or meat. If he eats a normal portion his pill doesn’t work. I can also see that the pill doesn’t work as well anymore as in the past.
Another problem he has is that when his pills stops working he has to go to the toilet. At night he is constantly going to the toilet which messes up his sleep, but also puts him in a dangerous situation because he can hardly walk at those times which can cause him to fall.
I can make a whole list of the issues he his having. I am pretty sure that with proper stem cells most of these issues would be solved or wouldn’t be so problematic anymore. So the faster these stem cells are ready, the better 🙂
LikeLike
I see your point. It is functionally a cure for the motor symptoms. However most PD patients end up in nursing home from dementia and cognitive symptoms.
That said, I don’t want to be dismissive of this stem cell treatment. It will be a massive massive step forward in the natural history of the disease. In many situations you may have the disease without the symptoms manifesting, and that is functionally a cure.
That said, Voyager’s AADC gene therapy is a competing approach to the stem cell based therapy, with similar efficacy expected. The only difference is that you will still need to continue taking a small dose of oral levodopa. I don’t think that’s a bad thing to do, considering that this approach lets you titrate your meds to the point of reaching maximum efficacy. If dopamine is the need, all really need to do is increase response to ldopa. As disease advances and there’s lesser and lesser enzyme in the nigrostriatal tract, levodopa response decreases. The gene therapy will confer the ability on the brain to convert levodopa to dopamine like it was the miracle drug in the early stages of the disease. Let’s not forget that the drug acts wonderfully well in the early stages, with almost no side effects.
LikeLike
Hi Deepak,
Thanks for your thoughts here – much appreciated. But if we look at cell transplantation as a cure in this manner, then we should look at levodopa as “functionally a cure”, and I am not prepared to do that. The word “cure” – which is an emotive word for many in the community – should only be used in terms of halting the disease (not symptoms) and permanently restoring function (removing dysfunction). Any other context and it gets folks upset. Trust me.
Kind regards,
Simon
LikeLiked by 1 person
Honestly I feel that gene therapies with external ldopa supplementation hold greater promise in the near future. They’re hopefully within reach too. Gene therapies have the potential to make the motor symptoms like insulin for diabetes. Basically pop in a pill and replenish your dopamine. They may have the same intended effect as cell replacement, with a long-term stable response to oral medication. Ask me and I’ll say that that’s not a bad trade off
LikeLike
A levodopa pill is not what you want. It interacts with proteins. My father has PD and this is one of his struggles. If he eats normally, his pills work late or don’t work at all. Also don’t forget that people with PD .. their stomach doesn’t work as well when they are having off periods. In my opinion, even if we don’t have stem cells yet, something most be found to deliver levedopa in a different way that avoids the digestive system.
LikeLiked by 1 person
I don’t disagree with any of that. There’s a large subset of PD patients for whom absorption isn’t a problem, but the bigger problem for them is that the absorbed drug doesn’t convert well into dopamine in the brain. This is because of the lack of AADC enzyme which erodes away with more and more neurons degenerating in the substantia nigra. So effectively replacing the AADC in the striatum can lead to an ectopic production of dopamine. In addition there are companies that are coming up with subcutaneous ldopa delivery. You have to look up neuroderm.
I agree with you that stem cells may be the long term option for a fix. However except for the Japanese, no one else has entered the clinic just yet. This is at least 5 years away if not more. Unless of course the FDA gives it fast track designation. The advantage with gene therapies and fast track is that gene expression is almost immediate. However these stem cell derived neurons take time to mature and it may be a while before we start seeing benefits
LikeLiked by 1 person